The molecular epidemiology of carbapenemase-producing Klebsiella pneumoniae (KPC) has been largely investigated, but limited clinical information is available. A case-control study was performed to evaluate the risk factors for KPC bacteremia in hospitalized patients. Cases were patients with KPC bacteremia and controls were patients with non-KPC bacteremia. A total of 85 patients were included, 18 (21.2%) were KPC, and 67 (78.8%) were non-KPC (40 [59.7%] of them were extended-spectrum beta-lactamase producers). All KPC isolates were type 2 producers. These isolates belong to five distinct clones. Multivariate analysis showed that age (odds ratio [OR], 1.06; 95% confidence interval [CI], 1.02 – 1.11; p = 0.004), presence of mechanical ventilation (OR, 11.1; 95% CI, 1.92 – 63.3; p = 0.007) and fluoroquinolone exposure during hospitalization (OR, 28.9; 95% CI, 1.85 – 454.6; p = 0.02) were independent risk factors for KPC in patients with K. pneumoniae bacteremia. Factors associated with severity of illness, such as age and mechanical ventilation, seem to be the main risks factors for KPC. Fluoroquinolones use might be a risk factor for KPC bacteremia. Further investigations on risk factors for KPC are warranted.
Klebsiella pneumoniae are major nosocomial pathogens, particularly those producing extended-spectrum beta-lactamase (ESBL).1 ESBL producers are usually susceptible only to carbapenems, and these drugs have been the treatment of choice for severe infections by ESBL-producer K. pneumoniae.1 More recently, the emergence of carbapenemase-producing Klebsiella pneumoniae (KPC) has severely challenged antimicrobial therapy, since it confers a high level of resistance to all beta-lactams and distinct levels of resistance to carbapenems.2
Although the molecular epidemiology of KPC isolates has been largely investigated, clinical aspects, including risk factors for infections by these organisms, have been poorly investigated so far.3,4 From a clinical and epidemiological perspective, identification of factors associated with KPC in patients with K. pneumoniae bacteremia is very important for both understanding the determinants of the dissemination of these organisms and potentially guiding empirical antimicrobial choice in severe infections by these organisms, such as bloodstream infections, which is critical for patient outcome.5 This study aimed to assess the risk factors for KPC in hospitalized patients with K. pneumoniae bacteremia.
MethodsStudy designA nested case-control study was performed at the Hospital Universitário Evangélico de Curitiba, a 660-bed tertiary-care hospital in Curitiba, Brazil. All patients ≥18 years of age with a positive blood culture with K. pneumoniae from January 2006 to August 2011 were included in the study. Only one episode per patient was analyzed. Data were collected from medical charts and/or hospital computerized system databases, except for case patients, whose data were prospectively collected. The study was approved by the local ethics review board.
Microbiological proceduresCultures were collected according to the standard protocol used in the hospital, and were processed using the BACT/Alert® (bioMérieux – Durham, USA). K. pneumoniae was identified by Vitek 2 (bioMérieux – Marcy’ Étoile, France). Susceptibility testing was performed by the disk diffusion method according to the CLSI guidelines.6 Detection of ESBL was determined by disk diffusion method according to CLSI recommendation.6 Isolates showing reduced susceptibility to ertapenem (zone diameter <20mm) were tested for detection of carbapenemase production using the modified Hodge test (MHT).6 Isolates with positive MHT were submitted to PCR for blaKPC using EasyQ KPC (bioMérieux – Marcy L’Étoile, France) with Escherichia coli ATCC 25922 and blaKPC-carrying K. pneumoniae strain ATCC BAA-1705 as negative and positive controls, respectively. The amplified product was purified using Exonuclease I and Shrimp Alkaline Phosphatase (ExoSAP, USB Corporatin) for DNA sequencing. Sequencing reactions were performed using BigDye v1.1 Sequencing Kits (Applied Biosystems – Foster City, CA, USA). Sequence data were acquired on ABI 3100 (Applied Biosystems – Foster City, CA, USA). The gene sequences were compared to entries in databases queried by NCBI BLAST (nucleotide sequence database).
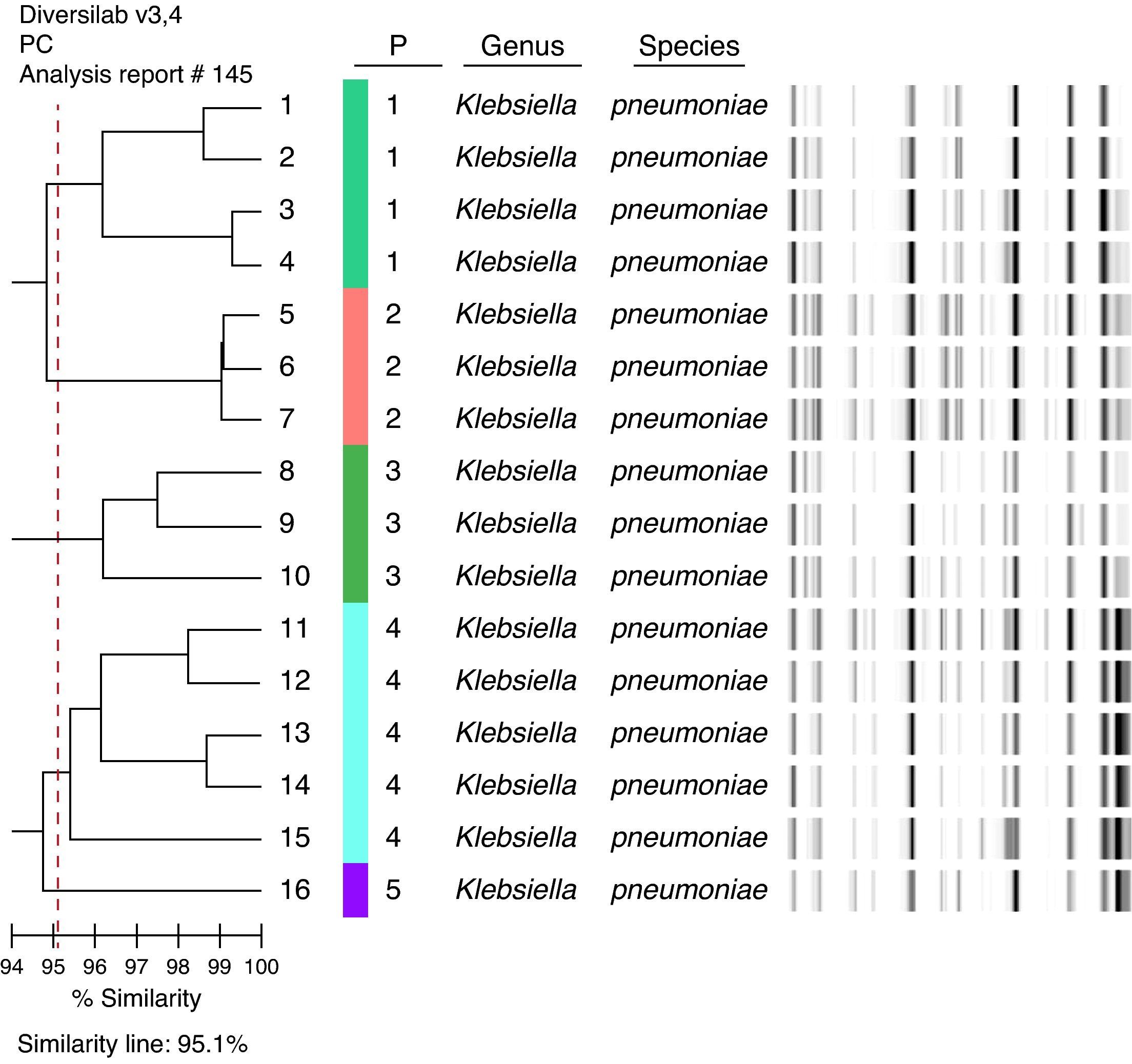
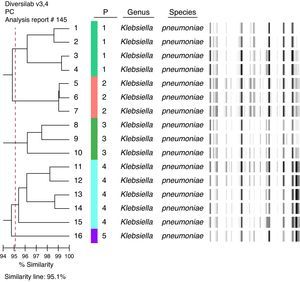
KPC isolates were examined using automated rep-PCR-based typing system (DiversiLabTM, bioMérieux – Athens, GA, USA).7 The results were analyzed and interpreted with the DiversiLab web-bases software using Pearson's correlation method. Clonally related isolates were defined as those with ≥ 95% homology.
Variables and definitionsCases were defined as patients with KPC bacteremia and controls were patients with non-KPC bacteremia. Potential risk factors assessed were: gender, age, previous hospital admission within the last 90 days, admission to the intensive care unit, length of hospitalization before bacteremia, use of mechanical ventilation, presence of central venous line and/or urinary catheter, surgery during current hospitalization, underlying comorbid conditions, and previous antibiotic use during current hospitalization.
Statistical analysisAll statistical analyses were carried out using the Statistical Package for Social Sciences (SPSS) for Windows, Version 18.0. Univariate analysis was performed separately for each variable. P-values were calculated using the chi-squared test or Fisher's exact test for categorical variables, and Student's t-test or Wilcoxon rank-sum test for continuous variables. Variables for which the p-value was ≤ 0.10 in the univariate analysis were included in a forward stepwise logistic regression model. Variables were checked for confounding and collinearity. A p-value of 0.05 was set as the limit for acceptance or removal of the new terms in the model. Goodness-of-fit was assessed by the Hosmer–Lemeshow test. All tests were two-tailed, and a p-value ≤ 0.05 was considered significant.
ResultsFrom a total of 932 modified Hodge tests performed in the period, 763 were true positives, 19 were false positives, 129 true negative, four were false negative, and 17 were inconclusive. This test showed 99.5% sensitivity and 87.1% specificity. The positive predictive value was 96.9% and the negative predictive value was 87.1%.
A total of 85 patients were included in this study. 18 patients (21.2%) had KPC bacteremia and 67 (78.8%) had non-KPC bacteremia (40 [59.7%] of them were ESBL-producers). All KPC isolates were type 2 producers. These isolates belong to five distinct clones: four isolates, clone 1; three isolates, clone 2; three isolates, clone 3; five isolates, clone 4, and one isolate, clone 5 (Fig. 1). Two isolates could not be typed.
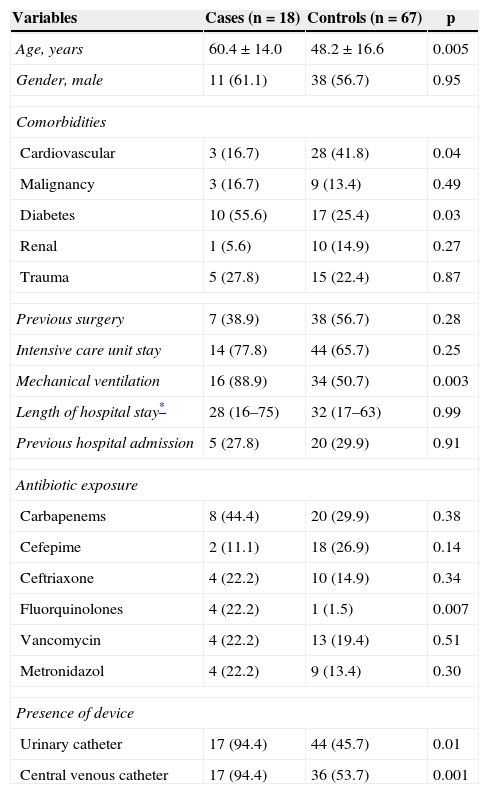
The univariate analysis for KPC bacteremia is presented in Table 1. Multivariate analysis showed that age (odds ratio [OR], 1.06; 95% confidence interval [CI], 1.02-1.11; p = 0.004), presence of mechanical ventilation (OR, 11.1; 95% CI: 1.92-63.3; p = 0.007), and ciprofloxacin exposure during hospitalization (OR, 28.9; 95% CI: 1.85-454.6; p = 0.02) were independent risk factors for KPC in patients with K. pneumoniae bacteremia (Hosmer-Lemeshow χ2 test = 4.266. p = 0.832).
Univariate analysis of risk factors for CP-producing Klebsiella pneumoniae bacteremia.
| Variables | Cases (n = 18) | Controls (n = 67) | p |
|---|---|---|---|
| Age, years | 60.4 ± 14.0 | 48.2 ± 16.6 | 0.005 |
| Gender, male | 11 (61.1) | 38 (56.7) | 0.95 |
| Comorbidities | |||
| Cardiovascular | 3 (16.7) | 28 (41.8) | 0.04 |
| Malignancy | 3 (16.7) | 9 (13.4) | 0.49 |
| Diabetes | 10 (55.6) | 17 (25.4) | 0.03 |
| Renal | 1 (5.6) | 10 (14.9) | 0.27 |
| Trauma | 5 (27.8) | 15 (22.4) | 0.87 |
| Previous surgery | 7 (38.9) | 38 (56.7) | 0.28 |
| Intensive care unit stay | 14 (77.8) | 44 (65.7) | 0.25 |
| Mechanical ventilation | 16 (88.9) | 34 (50.7) | 0.003 |
| Length of hospital stay* | 28 (16–75) | 32 (17–63) | 0.99 |
| Previous hospital admission | 5 (27.8) | 20 (29.9) | 0.91 |
| Antibiotic exposure | |||
| Carbapenems | 8 (44.4) | 20 (29.9) | 0.38 |
| Cefepime | 2 (11.1) | 18 (26.9) | 0.14 |
| Ceftriaxone | 4 (22.2) | 10 (14.9) | 0.34 |
| Fluorquinolones | 4 (22.2) | 1 (1.5) | 0.007 |
| Vancomycin | 4 (22.2) | 13 (19.4) | 0.51 |
| Metronidazol | 4 (22.2) | 9 (13.4) | 0.30 |
| Presence of device | |||
| Urinary catheter | 17 (94.4) | 44 (45.7) | 0.01 |
| Central venous catheter | 17 (94.4) | 36 (53.7) | 0.001 |
Values are number (%) or mean ± standard deviation unless otherwise indicated.
Carbapenemase producing (CP) Enterobacteriaceae have become endemic in many regions worldwide, especially among K. pneumoniae isolates.2 So far, ten types of CP have been described,8 but type 2 CP is by far the most frequently described.2 The molecular epidemiology of KPC has been broadly investigated and the worldwide dissemination of multiple clones, notably ST-258, has been clearly demonstrated.9 Additionally, knowledge on genes codifying these enzymes and surrounding genetic structures has also been advancing significantly.2 However, despite these advances, the clinical epidemiology of CP-producing organisms has been less investigated. To date, only one study has investigated the risk factors for KPC,4 and another has investigated the risk factors for KPC and metallo-beta-lactamases-producing K. pneumoniae bacteremia.3 In this study, the risk factors for KPC were assessed in patients with K. pneumoniae bloodstream infections.
This study found that advanced age, mechanical ventilation, and previous exposure to ciprofloxacin were independently associated with increased risk for KPC in this population.
Advanced age has not been found as a risk factor for KPC in previous studies, but it has been shown that severity of illness, as determined by the All Patient Refined-Diagnosis Related Group method or by the APACHE II score,3 was independently associated with KPC recovery and bacteremia, respectively. Since it was not possible to assess any score of severity of illness as a potential risk factor, age is believed to have been a surrogate marker of such condition, as it might also be in the case of mechanical ventilation. Interestingly, no previous study has found carbapenems exposure as a risk factor for KPC.3,4 Indeed, it was forced into the final multivariate model without modifying the results (data not shown). Actually, Mouloudi et al. found no antimicrobial class exposure as a risk factor for KPC bacteremia.3 Conversely, Gasink et al. found that prior use of an extended-spectrum cephalosporin and ciprofloxacin were both independently associated with KPC.4
Fluoroquinolones (all received ciprofloxacin, data not shown) were found to be an independent risk factor, which might be explained by the fact that plasmid-encoded qnr genes, which determine low-level fluoroquinolone resistance, have been identified in the same conjugative K. pneumonia plasmid as CP genes (specifically blaKPC-2 and qnrB4).10 Although this factor remained independently associated with outcome and is corroborated by a previous study, caution is recommended when interpreting and generalizing the present findings, since the exposure rate to this drug in control patients was extremely low (1.5%).
This study has some limitations that must be acknowledged. First, data from control patients were retrospectively recovered, and some important variables such as scores for severity of illness could not be precisely assessed. However, as discussed above, age and mechanical ventilation are believed to be surrogate markers of severity, which seems to be an important risk for KPC. Additionally, although many variables were associated with the outcome in univariate analysis, the number of patients included in the study might have reduced the statistical power to detect minor differences between groups for some variables. Finally, carbapenem exposure may have not been found as a risk for KPC bacteremia because previous antimicrobial exposure was considered during the entire admission period. Thus, it is possible that numerous control patients received a carbapenem drug many days before the recovery of K. pneumoniae from blood. In fact, recent exposures to antimicrobials are usually more frequently associated with the recovery of resistant bacteria.11
In summary, this was the second study designed to assess risk factors for KPC bacteremia. Factors associated with severity of illness, such as age and mechanical ventilation, seem to be the main risks factors for KPC, as also indicated by previous studies. Fluoroquinolones might be a risk factor for KPC bacteremia. Further investigations on risk factors for KPC are required to better understand the epidemiological determinants of the dissemination of these organisms and to optimize empirical therapy for high-risk group in settings with high prevalence of KPC.
Conflict of interestFelipe F. Tuon received grants from Merck, Pfizer, Novartis, and United Medical (Gilead). Jaime L. Rocha received grants from Merck, Pfizer, Novartis, Sanofi, and AztraZeneca. Paula V. M. Toledo received grants from Tibotec, Roche, Bristol Myers Squibb, GlaxoSmithKlein, and bioMérieux. Alexandre P. Zavascki is a research fellow from the National Council for Scientific and Technological Development (CNPq), Ministry of Science and Technology, Brazil (301829/2008-0).
The authors would like to thank the HAI Control Division from Municipal Department of Health for data of the current outbreak and the Bacteriology and Molecular Biology Group of the LACEN-PR.