Patients with kidney disease on Hemodialysis (HD) are susceptible to Coronavirus Disease (COVID-19) due to multiple risk factors.
AimThis study aims to report the prevalence of antibodies against SARS-CoV-2 among patients on hemodialysis before vaccination in Brazil and to compare with clinical, demographic, and laboratory data.
MethodsBlood samples from 398 Chronic Kidney Disease (CKD) patients treated in three different private institutions in Rio de Janeiro State, Brazil were submitted to the total anti-SARS-CoV-2 testing. Kidney, liver, and hematological markers were also determined. Respiratory samples were tested by real-time PCR for SARS-CoV-2 RNA and positive samples were subjected to high-throughput sequencing on the MinION device.
ResultsOverall, anti-SARS-CoV-2 prevalence was 54.5 % (217/398) and two individuals had SARS-CoV-2 RNA with variant B.1.1. High anti-SARS-CoV-2 seroprevalence was found in male gender and those with hospital admission in the last 3-months before the inclusion in the study. Lower red blood cell count was observed in the anti-SARS-CoV-2 seropositive group. High levels of anti-SARS-CoV-2 were found in those who reported symptoms, had low levels of eosinophils and low hematocrit, and who practiced physical activity.
ConclusionHigh prevalence of anti-SARS-CoV-2 was found in CKD patients before the universal immunization in Brazil suggesting that dialysis patients were highly exposed to SARS-CoV-2.
On March 11, 2020, the World Health Organization (WHO) characterized COVID-19 as a pandemic disease caused by the Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2), after 118,000 cases and 4291 deaths reported in 114 countries.1 On September 28, 2020 (date referring to the first collection of this study) over 32.7 million cases of COVID-19 and 991,000 deaths were reported by WHO.2 By the week of November 5 (the second collection of our study), the numbers accumulated to over 49.7 million reported cases and over 1.2 million deaths worldwide.3 By the week of January 19, 2021 (the last collection date of our study) cases have reached over 98.2 million cases and over 2.1 million deaths worldwide.4
It has been observed that Chronic Kidney Disease (CDK) patients are at over 4 fold increased mortality risk compared to healthy individuals.5 CDK leads to marked immunosuppression and has been associated with poor COVID-19 outcomes.6 Risk factors as hypertension, diabetes, obesity, elderly age, cardiovascular disease have also been associated to increase of COVID-19 severity.7,8 According literature, the HD patients presented 10 %‒50 % of asymptomatic COVID-19 infections.6 Renal patients on hemodialysis have a higher risk of transmission. Normally, they attend clinics three times a week, having frequent contact with doctors, nurses and other patients on their dialysis. Even with strict protocols and specific recommendations, many infections are under risk among these individuals.9
During the pandemic, several Variants of Concern (VOCs) have emerged, which are identified by their high transmissibility and their ability to evade innate and acquired immune responses by vaccination.5,10 Between late 2020 and early 2021, four major VOCs emerged worldwide: B.1.1.7 (Alpha), B.1.351 (Beta), P.1 (Gamma) and B.1.617.2 (Delta).10,11 The first wave had the predominance of the variant B.1.1.33, being identified in Brazil between April and May 2020. During this wave, 99,760 cases were reported, with 24,174 hospitalizations and 11,270 deaths. The 2nd wave occurred between November 2020 and January 2021, where P.2 (Zeta) was predominant. These isolates were first detected in Rio de Janeiro and then in the other Brazilian states. In this period, 282,339 cases were reported, 27,778 hospitalizations, and 10,621 deaths.11,12
Serological testing is paramount to investigate patient's previous contact with the virus or the development of long-lasting immunity.13 Individuals on hemodialysis have an affected humoral immune response, leading to lower seroconversion and decrease in antibodies compared to healthy individuals.14
It has been observed that individuals undergoing HD constitute a population susceptible to severe COVID-19. Accessing SARS-CoV-2 seroprevalence in a cohort of patients with CKD on HD is important to a better understanding of their natural immunity. The main question of the study was to assess SARS-CoV-2 prevalence in CKD patients and its influence on clinical and laboratorial aspects of kidney damage.
Materials and methodsStudy populationThis cross-sectional study included 398 CKD patients undergoing HD treatment (level 5) in three different private institutions in Rio de Janeiro State (Brazil), from September 2020 to January 2021, during the end of COVID-19 first wave and the beginning of the second wave. These HD units were in the following municipalities in Rio de Janeiro State: (i) Rio de Janeiro City (State capital; n = 171), (ii) Japeri (70 km from the State capital; n = 117), and (iii) Queimados (45 km from the State capital; n = 110).
Inclusion criteria comprised individuals who agreed to sign the Informed Consent Form (ICF) and who were under active dialysis treatment. Exclusion criteria included limitations in understanding ICF terms and insufficient sample volume for posterior tests. Demographic data, clinical characteristics, and risk factors were collected through a questionnaire application. Clinical data and risk factors were not obtained at the Rio de Janeiro unit. Volunteers were argued about their physical activity where it was considered adequate when they reported average weekly volumes of 150–300 min of moderate intensity. Clinical manifestations were also assessed by questionnaire where they should report respiratory symptoms or other clinical manifestations before inclusion the study. The current study was conducted in compliance with the Declaration of Helsinki; it was approved by the National Ethics Committee of Brazil (CONEP) under CAAE number 34049514.7.0000.5248.
Sample collectionParticipants underwent blood and intranasally/oropharyngeal swab collection. Blood was collected by peripheral venipuncture based on using hypodermic needles and sterile 8.5 mL gel vacutainer tubes (SSTTM II Advance, BD Vacutainer®, USA). Serum was stored at −20 °C. Two swabs per patient were collected from nasal (1) and oropharyngeal (1) sites. After the collection procedure was over, swabs were placed in 0.9 % saline (NaCl) solution. The material was aliquoted and stored at −70 °C until testing time.
Serological assaysSerum samples were tested for total anti-SARS-CoV-2 antibodies through Elecsys Anti-SARS-CoV-2 qualitative immunoassay (Roche Diagnostics, Basel, Switzerland). This test uses a recombinant protein that corresponds to SARS-CoV-2 nucleocapsid protein; it was performed in Cobas and in the e801 platform (Roche diagnostics). Reactive samples showed a relative Cut-Off Index (COI) higher than 1.0. According to the manufacturer, test specificity and sensitivity reached 99.81 % and 100 %, respectively, in cases showing more than 14-days of RT-qPCR positivity. Springer et al.15 demonstrated the highest sensitivity for detecting nucleocapsid specific antibodies against SARS CoV-2 compared to other assays and Di Meo et al.16 also used the same assay to measure anti-SARS CoV-2 in hemodialysis patients with good sensitivity.
Molecular assays for SARS CoV-2 RNA detectionSwab samples were submitted to SARS-CoV-2 RNA extraction using QIAamp Viral RNA Mini Kit (QIAGEN, Hilden, Germany), as well as to quantitative Reverse Transcription Polymerase Chain Reaction (RT-qPCR) added with a set of probe-associated primers (assay) aimed at SARS-CoV-2 nucleocapsid (N1 and N2) and Envelope (E) genes.17,18 Ribonuclease P/MRP Subunit P30 (RPP30) detection was carried out as endogenous control. The reaction was performed with AgPath-ID™ One-Step RT-PCR (Thermo Fisher Scientific, Waltham, USA) in Rotor Gene Plex-5 equipment (QIAGEN). Negative Template Control (NTC) was added to the extraction procedure; two negative synthetic controls for SARS-CoV-2 (SARS-CoV and MERS-CoV) were included in each RT-qPCR procedure to help monitoring RNA extraction and RT-qPCR quality.
RT-qPCR reaction conditions were initially performed at 45 °C for 15 min (reverse transcription); it was followed by 95 °C for 10 min (initial denaturing), and by 45 cycles of 95 °C for 15 s, and of 55 °C for 45 s. All samples were tested in duplicate. Fluorescence readings were detected at FAM channel. Cycle threshold (Ct) values were automatically provided in each run. Simultaneous Ct values lower than 40, for 2 out of 3 genes, represented positive results.
SARS-CoV-2 lineage genotyping was carried out by high-throughput sequencing via MinION device (Oxford Nanopore Technologies, Oxford, United Kingdom). Initially, SuperScript™ IV First-Strand Synthesis System (Thermo Fisher Scientific, Waltham, Massachusetts, USA) was used to reverse transcription. Next, PCR amplification of SARS-CoV-2 complete genome with two separate pools of primers19 has been carried out using Q5 Hot Start High-Fidelity DNA Polymerase (New England Biolabs, Massachusetts, USA). Subsequently, end-repair and dA-tailing was performed with commercial reagent NEBNext Ultra II End Repair/dA tailing module (New England Biolabs). For native barcode ligation, a mixture with end prep products, native barcodes (EXP-NBD104 and EXP-NBD114, Oxford Nanopore Technologies, Oxford, UK) and Blunt/TA Ligase master mix (New England Biolabs) was prepared. Pooled barcoded libraries were purified using ProNex® Size-Selective Purification System (Promega, Madison, WI, USA), quantified by fluorometer Qubit dsDNA HS Assay Kit (Thermo Fisher Scientific), and used for adaptor insertion with an NEBNext Quick Ligation Module (New England Biolabs). Reagents from Ligation Sequencing Kit (SQK-LSK109, Oxford Nanopore Technologies) were used to result in an eluted sequencing library. A primed R9.4.1 flow cell (FLO-MIN106D) was used to load the library and was sequenced on a MinION Mk1B device.
Evaluation of the hematological and the biochemical markersBlood samples were sent to central laboratory of Federal Hospital of Servers of the State in Rio de Janeiro, where biochemical tests were performed for evaluation of liver function, with dosage of the following enzymes: Alanine Amino Transferase (ALT), Aspartate Amino Transferase (AST), Gamma Glutamyl Transferase (GGT), Alkaline Phosphatase (ALP), Total Bilirubin (BT) and its fractions [Direct Bilirubin (BD), and Indirect Bilirubin (BI)]. All tests were performed using the dry chemistry analysis methodology through the equipment Clinical Chemistry Analyzer AU680 (Beckman Coulter, California, USA).
Based on the Electrical Impedance method, data from complete blood (hematocrit, hemoglobin, leukocytes, red blood cells, eosinophils, basophils, neutrophils, lymphocytes and monocytes), and platelet count was performed using the Coulter LH 750 Hematology Analyzer (Beckman Coulter).
Kidney function biochemical parameters, such as urea, creatinine, phosphorus, and calcium were measured. Urea was measured by UV Enzymatic methodology, creatinine by Jaffe Colorimetric-Kinetic method, phosphorus by Colorimetric-Phosphomolybdate (PVP) methodology and calcium by O-cresolphthalein method.
Statistical analysisDemographic, clinical, laboratory and epidemiological data were inserted in electronic spreadsheet in Excel® software, version 2019. Relative and absolute frequencies observed for biochemical, hematological, sociodemographic, and behavioral profiles were measured based on using chi-square test for the homogeneity of categorical variables and Student's t-test for continuous variables. Variables showing p-value < 0.05 in the homogeneity Chi-Square test and in Student's t-test during the modeling process were selected for the multivariate model. Analyses were performed in the Statistic Package for Social Science software (SPSS for Windows, version 21.0). In addition, mean antibody levels were compared between groups by taking into consideration the investigated variables as well as their significance after multivariate analysis; Mann-Whitney U test was performed at significance level corresponding to p < 0.05.
ResultsSARS CoV-2 serological and molecular prevalenceIn total, 217 (54.5 %) individuals in the investigated sample (n = 398) had IgG/IgM anti-SARS-CoV-2 detected in serum and 2 individuals (0.8 %) had SARS-CoV-2 RNA detected in swab samples. With respect to molecular results, SARS-CoV-2 N1, N2 and E genes’ Ct values recorded for the two patients detected in the SWAB reached 36.85, 39.17, 42.81, and 23.37, 24.56, 23.96, respectively. One of the aforementioned patients was from the Japeri unit, whereas the other one came from Queimados. Sequencing was only possible in the sample collected from the patient treated at the Japeri HD unit; this patient was the one presenting variant B.1.1.
Anti-SARS-CoV-2 prevalence according demografic characteristics and clinical factors in this study, mean age of the individuals was 52.1 ± 15.2 years and most of them was male 216/398 (54.3 %) and African ethnicity 228/313 (72.9 %). Mean time at hemodialysis treatment was 49.2 months.
Information about the clinical characteristic were obtained in the Japeri and Queimados units. Regarding the clinical factors, 72.4 % of individuals reactive for SARS-CoV-2 did not report any respiratory symptoms before the inclusion in the study. Regarding the physical activity, 106/2017 (48.9 %) did not report the minimum of physical activity recommended by WHO guidelines (average weekly volumes of 150–300 min of moderate intensity).
Hospitalization was more frequent among individuals who anti-SARS-COV-2 reactive compared to non-reactive patients (24 vs. 8) and 25 % of these hospitalized patients (6/24) were known to be due to respiratory infection caused by COVID-19.
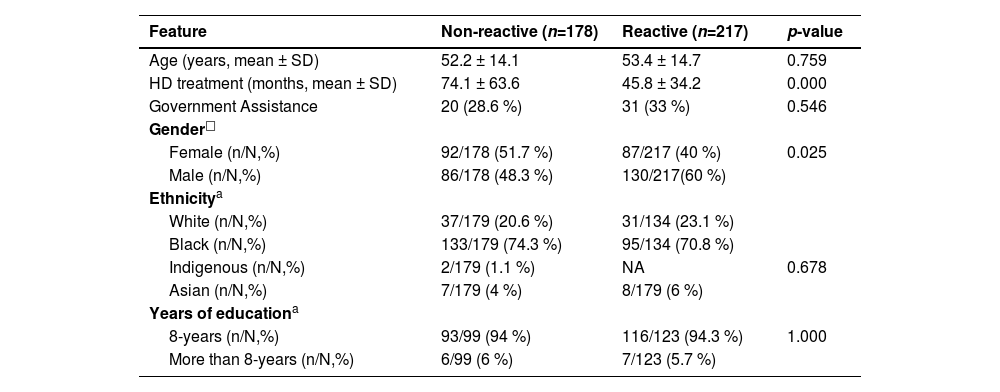
Hypertension was the most frequent comorbidity (156/226 %–69.1 %), followed by diabetes (51/226 %–22.5 %). Table 1 shows the bivariate analysis of anti-SARS-CoV-2 prevalence according to sociodemographic factors where male gender (p = 0.025) and lower mean time at hemodialysis treatment (p = 0.000) was associated to high anti-SARS-CoV-2 positivity.
Bivariate analysis of sociodemographic characteristics in relation to seropositivity to anti-SARS-CoV-2.
| Feature | Non-reactive (n=178) | Reactive (n=217) | p-value |
|---|---|---|---|
| Age (years, mean ± SD) | 52.2 ± 14.1 | 53.4 ± 14.7 | 0.759 |
| HD treatment (months, mean ± SD) | 74.1 ± 63.6 | 45.8 ± 34.2 | 0.000 |
| Government Assistance | 20 (28.6 %) | 31 (33 %) | 0.546 |
| Gender□ | |||
| Female (n/N,%) | 92/178 (51.7 %) | 87/217 (40 %) | 0.025 |
| Male (n/N,%) | 86/178 (48.3 %) | 130/217(60 %) | |
| Ethnicitya | |||
| White (n/N,%) | 37/179 (20.6 %) | 31/134 (23.1 %) | |
| Black (n/N,%) | 133/179 (74.3 %) | 95/134 (70.8 %) | |
| Indigenous (n/N,%) | 2/179 (1.1 %) | NA | 0.678 |
| Asian (n/N,%) | 7/179 (4 %) | 8/179 (6 %) | |
| Years of educationa | |||
| 8-years (n/N,%) | 93/99 (94 %) | 116/123 (94.3 %) | 1.000 |
| More than 8-years (n/N,%) | 6/99 (6 %) | 7/123 (5.7 %) |
N, Total of individuals in this specific analysis; n, Total of patients within N; NA, Does Not Apply, means no result (p-value < 0.053).
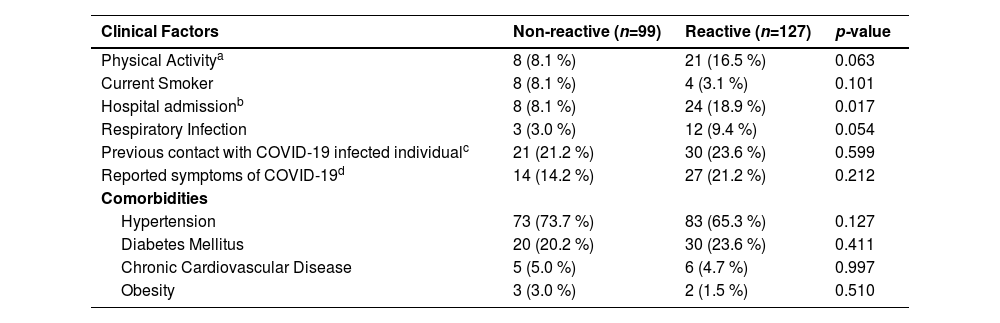
At bivariate analysis, hospital admission in the last 3-months before the inclusion in the study was associated with anti-SARS-CoV-2 prevalence (p = 0.017) where most of the reactive individuals reported this hospitalization. The presence of respiratory infection was also frequent among reactive individuals (p = 0.05) although it was not significant. On the other hand, there was no significant difference between the risk factors (hypertension, diabetes, cardiovascular disease, obesity) for seropositivity (Table 2).
Bivariate Analysis of clinical and risk factors in relation to seropositivity to anti-SARS-CoV-2 in patients with CKD, who were treated at Japeri and Queimados units. 2020 and 2021 (n = 226).
| Clinical Factors | Non-reactive (n=99) | Reactive (n=127) | p-value |
|---|---|---|---|
| Physical Activitya | 8 (8.1 %) | 21 (16.5 %) | 0.063 |
| Current Smoker | 8 (8.1 %) | 4 (3.1 %) | 0.101 |
| Hospital admissionb | 8 (8.1 %) | 24 (18.9 %) | 0.017 |
| Respiratory Infection | 3 (3.0 %) | 12 (9.4 %) | 0.054 |
| Previous contact with COVID-19 infected individualc | 21 (21.2 %) | 30 (23.6 %) | 0.599 |
| Reported symptoms of COVID-19d | 14 (14.2 %) | 27 (21.2 %) | 0.212 |
| Comorbidities | |||
| Hypertension | 73 (73.7 %) | 83 (65.3 %) | 0.127 |
| Diabetes Mellitus | 20 (20.2 %) | 30 (23.6 %) | 0.411 |
| Chronic Cardiovascular Disease | 5 (5.0 %) | 6 (4.7 %) | 0.997 |
| Obesity | 3 (3.0 %) | 2 (1.5 %) | 0.510 |
Hematological and biochemical markers were investigated in Queimados and Japeri units due to sample volume availability. In this population, 85.3 % of individuals presented low levels of hemoglobin and 58.3 % of them had antibodies against SARS-CoV-2. Regarding anti-SARS-CoV-2 reactive patients, we also found nine individuals with leukopenia and eight with leukocytosis.
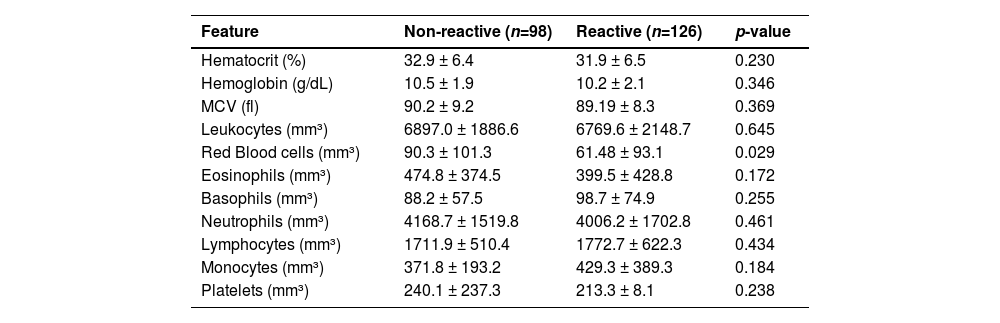
Table 3 demonstrates a bivariate analysis of anti-SARS-CoV-2 positivity according to hematological markers. Patients reactive for anti-SARS-CoV-2 had lower mean values of red blood cells compared to the non-reactive group (p = 0.029). Even there was no found significance in white blood cell counts considering the seropositivity investigation, low counts of eosinophils (474.8 vs. 399.5) as well as high counts of monocytes (371.8 vs. 429.3) and basophils (88.2 vs. 98.7) were observed in the group of anti-SARS-CoV-2 seropositive patients (Table 3).
Hematological profile regarding total antibody positivity for SARS-CoV-2 in patients with CKD, who were treated at Japeri and Queimados units. 2020 and 2021 (n = 227).
MCV, Mean Corpuscular Volume. p-value < 0.05.
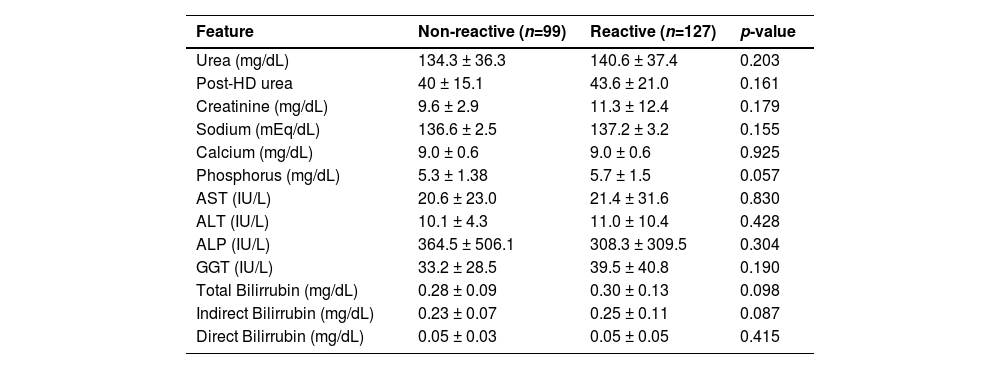
We also investigated the levels of biochemical markers according to anti-SARS-CoV-2 prevalence and none of these parameters was statistically significant as shown in Table 4. However, anti-SARS-CoV-2 reactive patients had elevated values of urea (134.3 vs. 140.6;) and creatinine (9.6 vs. 11.3) considering the reference values for healthy subjects (healthy reference values – urea: 15‒45 mg/dL; creatinine: 0.5‒1.3 mg/dL). It was also found that patients had 3-fold elevated Alkaline Phosphatase (ALP) levels, if compared with reference values for healthy subjects (reference value: 34‒104 U/L).
Biochemical profile in patients with pre-existing chronic kidney disease based on total anti-SARS-CoV-2 reactivity, at Japeri and Queimados units. 2020 and 2021 (n = 226).
HD, Hemodialysis; AST, Aspartate Aminotransferase; ALT, Alanine Aminotransferase; ALP, Alkaline Phosphatase; GGT, Gamma Glutamyl Transferase.
The data from anti-SARS-CoV-2 detection displayed the amount of bioluminescence by a luminometer and the result is given in units known as the Relative Light Units (RLU). Considering that, the RLU were used to group comparisons based on the relevant data of clinical and laboratory findings.
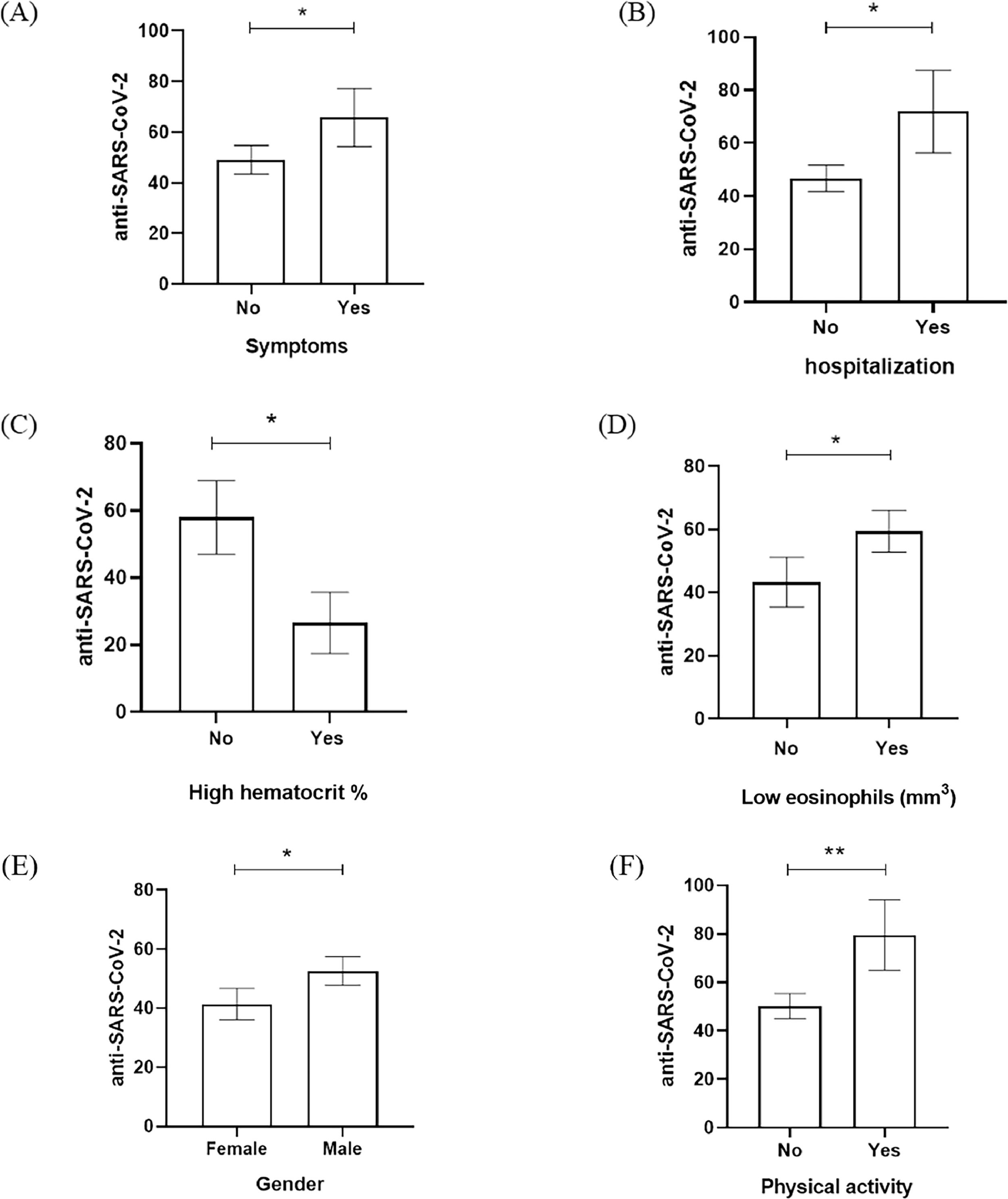
According to that, variables such as the symptoms, hospitalization admission, hematocrit, eosinophils, gender, and physical activity have seemed to be significantly related to RLU data from anti-SARS-CoV-2 detection (Fig. 1). Fig. 1 showed that the presence of COVID-19 symptoms (dry cough, headache, body pain, sore throat, fatigue, and respiratory distress), hospitalization admission, and physical activity had significant elevation of anti-SARS-CoV-2 RLU in comparison with patients who were asymptomatic, no hospitalization and no physical activity (Fig. 1A, 1B, 1F). Male gender was the predominant population in our study and also had a significant increase of anti-SARS-CoV-2 RLU in comparison to the female group (Fig. 1E). When the laboratory data was evaluated, the low percentage of hematocrit and low eosinophils counts in the study population was anti-SARS-CoV-2 RLU elevated (Fig. 1C, 1D).
Anti-SARS-CoV-2 detection (RLU) based on the relevant variables investigated in the assessed population. (A) Presence of symptoms, (B) hospitalization admission, (C) high hematocrit percentage (%) (D) low eosinophils count (mm3), (E) gender, and (F) physical activity according to anti-SARS-CoV-2 RLU detection. * p-value < 0.01; ** p-value < 0.001.
This is a cross-sectional study conducted in Rio de Janeiro state, Brazil, to assess the seroprevalence of SARS-CoV-2 in individuals with CKD who were under HD treatment during the end of the first wave and the beginning of the second wave of the COVID-19 pandemic in Brazil. High prevalence of anti-SARS CoV-2 was found in CKD patients and statistically associated to hospitalization in the last 3-months, female gender, and low mean time (in months) at hemodialysis treatment. In addition, these individuals should be at a dialysis center at least 3 times per week where they are exposed to other individuals who could be infected.
A significant difference in antibody levels between men and women was found in the current study; men had higher antibody levels than women. According to the literature, men are more affected by severe illness and more likely to die from severe acute respiratory syndrome than women; this finding suggests sex-based differences in patients’ immune responses during COVID-19 infection.20,21 Accordingly, male individuals presented a higher frequency of non-classical monocytes associated with a lower frequency of T-cells. This feature was reactive correlated to CCL5 levels; this chemokine plays a key role in T-cells’ recruitment to inflammatory sites.20,21 According to another study, male patients presented higher antibody levels than female patients, and it has contributed to autoantibody production, which led to severe disease symptoms and death. A study recorded male-sex bias during the recovery stage of patients with mild COVID-19 symptoms since men produced more robust anti-SARS-CoV-2-spike protein and higher neutralizing antibodies than women.21
Hypertension and diabetes were the comorbidities most often observed in the current study, as previously shown in other cohorts of CKD patients, regardless of SARS-CoV-2 infection.22,23 These comorbidities have been seen as the leading causes of COVID-19-associated death in patients with CKD.24 Hypertensive nephropathy (50 %) was the most common cause of CKD; it was followed by diabetic nephropathy (20.9 %). Based on a study conducted with HD and COVID-19 patients in China, diabetic nephropathy (26.7 %) and hypertensive kidney disease (26 %) were the main primary causes of CKD.25
The current study recorded a high prevalence of SARS-CoV-2 (54.5 %) antibodies in CKD patients within the investigated period of time in comparison to other studies conducted with this population (5.17 %). On the other hand, anti-SARS-CoV-2 prevalence in CKD patients ranged from 15 % in China,24 28 % in the USA,26 and 36.2 % in the United Kingdom (UK), within this very same period.27
The current study observed a low prevalence (2/227 %‒0.8 %) of active SARS-CoV-2 infection with viral RNA detection at RT-qPCR in comparison to studies conducted with dialysis patients in the UK (22.2 %),24 China (53 %),27 Italy (15 %)28 and London (19.6 %).22 This finding may be associated with the time sample collection was performed, likely 14 days after SARS-CoV-2 infection, when RNA is not often detected by molecular tools. In our study, we detected the ongoing SARS-CoV-2 in two patients, but it was possible to sequence only one individual, this individual was infected in the period January 2021 and the variant detected was the B.1.1, in this period Brazil was already in the 2nd wave, which started between November 2020 and January 2021, where the P.2 (Zeta) was predominant. The P2 variant originated from the B.1.1.28 lineage.11,12
Hematological parameters may have a major impact on SARS-CoV-2 infection in course in individuals with CKD. The present study has found that individuals showing anti-SARS-CoV-2-reactive serology presented abnormal hematological parameters, such as low mean eosinophil levels and high mean monocyte rates. Low hemoglobin levels were reported in 55.5 % (216/227) of individuals with anti-SARS-CoV-2. Hemoglobin levels have significantly decreased in COVID-19 patients with severe disease symptoms.23 Furthermore, overall, CKD patients under HD treatment present significantly decreased hemoglobin levels; they are often anemic patients.29 Low hemoglobin level was associated with cardiovascular changes, worsened quality of life, and increased hospitalization and mortality rates.29
HD patients should often have their biochemical markers monitored. Changes in urea, creatinine, hemoglobin, phosphorus, potassium, and calcium levels have been described as common findings in CKD patients.30 The present study recorded high mean urea and creatinine levels for anti-SARS-CoV-2-reactive patients as findings observed for 40 % of individuals with COVID-19 in a study conducted in China.31 The current findings have indicated changes in GGT, total bilirubin, and indirect bilirubin levels in anti-SARS-CoV-2-reagent individuals. Data from previous studies have shown that COVID-19 patients may have liver dysfunction and present increased GGT, aminotransferases, bilirubin, and alkaline phosphatase levels.30,32
Based on the herein analyzed symptoms, results have suggested that symptomatic patients produced more antibodies than the asymptomatic ones. In addition, neutralizing activity was correlated to symptom duration and severity during SARS-CoV-2 infection.33 Moreover, patients with mild COVID-19 symptoms presented increased serum titers of S1-specific IgA and IgG.34
Considering our findings, individuals presenting low antibody levels have shown high eosinophil levels. On the other hand, other studies have shown that low eosinophil levels were associated with disease severity and clinical outcomes.34 Patients with severe COVID-19 have shown reduced eosinophils level in peripheral blood, although they recorded a high frequency of eosinophils in the lungs.35–37
Laboratory test results have emphasized that individuals presenting low hematocrit levels recorded higher anti-SARS-CoV-2 IgG levels, such as the ones observed in previous research conducted by Ouyang and collaborators (2020), who recorded lower hematocrit levels in both surviving and non-surviving COVID-19 patients, regardless of high antibody levels observed in them.38
In the present study, patients seropositive to SARS-CoV-2 were hospitalized more often compared to non-reactive patients and had higher levels of anti-SARS-CoV-2. In 2020, Lano e collaborators reported that 81 % of dialysis patients were found to be hospitalized due to COVID-19.6 This finding could be the result of the depressed immune system and social vulnerability of these patients that could favor the exposition to the virus.
In this present study, anti-SARS-CoV-2 levels were evaluated according to different variables. High levels of antibodies were associated with physical activity. Several reports about the health benefits of exercising have been published; thus, they can help individuals avoid many diseases, mainly inflammatory ones.33,34 It has been suggested that moderate exercising activates the immune system by triggering cellular and humoral immune responses.33,35 Exercising releases cytokines and neutrophils due to cortisol influence; besides, it increases circulating lymphocyte concentrations, which favor Th1-mediated immune response, antibody production, and viral elimination.39 Exercising on a regular basis can help strengthen the immune system functions and enable faster immunological responses against microorganisms.40
Some limitations should be considered here: (i) The impossibility of collecting more detailed information; (ii) Missing data in questionnaires applied in Rio de Janeiro unit, and swab samples deriving from the dialysis center in the Rio de Janeiro City; (iii) And the absence of comparison of the seroprevalence of the study participants with that of the general population or with that of people not seeking care in dialysis clinics. However, this transverse cross-sectional study provided information about the epidemiological and clinical features of HD patients with COVID-19 in Rio de Janeiro, Brazil, before immunization.
ConclusionsIn general, the patients who had higher antibody titers were those who had been hospitalized and were symptomatic, compared to those who had not been hospitalized and were asymptomatic. Also, patients with higher antibody titers had lower eosinophil levels. In general, serological investigation of antibodies is of great importance to assess the transmission characteristics of SARS-CoV-2 in different populations. Even with all the progress made, many questions about the humoral immune response to SARS-CoV-2 remain unanswered. Mainly about the intrinsic factors that influence the uncontrolled humoral immune response in some patients that lead them to develop severe disease.
Author's contributionConceptualization, data curation and formal analysis were performed by AC and LVM. Investigation, software analysis and laboratory assays were performed by AC, VD, JC, LL, FF, RJ, AM and GP. Writing, editing, and review were performed by AC, VSP, BVL, VD, JGM and LVM. Funding acquisition was obtained by LVM (INOVA FIOCRUZ VPPCB-005-FIO-20-2-52 grant #484015522386498; Carlos Chagas Filho Foundation for Research Support of the State of Rio de Janeiro (FAPERJ) grant numbers E26/204.196, E-26/204.197/2021, and E-26/200.821/202; the Higher Education Personnel Improvement Coordination (CAPES) grant numbers 88887.506556/2020-00, and the National Council for Scientific and Technological Development (CNPq) grant number 315967/2021-8.) and JGM (Faperj grant #E-26/200.205/2023).
FundingThe current study received financial support from Oswaldo Cruz Foundation, FAPERJ, CAPES and CNPq.
Ethical approval and consent to participateThis study was conducted in compliance with the Declaration of Helsinki; it was approved by the National Ethics Committee of Brazil (CONEP) under CAAE number 34049514.7.0000.5248 and approval number 4.112.243. Informed consent form was signed by all participants.
This study had the collaboration of several professionals. Juliana Custódio Miguel, Elisangela Ferreira da Silva, Giselle Prado do Nascimento and Julia Trece Marques helped with the serological tests. All authors critically reviewed the manuscript for important intellectual content and approved the final version for publication. We also would acknowledge the hemodialysis clinics in the state of Rio de Janeiro and the volunteers who made this study possible.