Sepsis is a systemic inflammation associated with infection caused by pathogenic micro-organisms with high mortality rates.
ObjectiveIn this study, we investigated the protective effect of Propionibacterium acnes-killed against polymicrobial sepsis induced by cecal ligation and puncture.
MethodsThe mice were treated by intramuscular route in 1, 3, 5, and 7 days before the cecal ligation and puncture induction. The control group animals received vehicle (saline solution 0.9%) and the animals of the treated group received the P. acnes-killed (0.4mg/animal). After anesthesia, midline laparotomy was performed with exposure of cecum followed by ligature and one transverse perforation of the same, with a 18G needle, for induction of lethal sepsis. After surgery, the cecum of the animals was replaced into the peritoneal cavity, and it was closed with a 4.0 nylon suture. The survival of animals subjected to lethal sepsis was evaluated after cecal ligation and puncture induction. Six hours after the induction of sepsis, neutrophil migration, the number of bacteria, TNF-α, MCP-1, IL-6, and IL-10 were performed in the peritoneal lavage.
ResultsProphylactic treatment with P. acnes-killed increased the survival of the animals, followed by a significant decrease in the TNF-α, IL-10, and MCP-1 levels, 6h after cecal ligation and puncture. Furthermore, P. acnes-killed administration reduced the number of bacteria in the peritoneal cavity with increased migration of leukocytes, especially neutrophils.
ConclusionP. acnes-killed promoted increased survival rate of animals with sepsis, in part attributed to its immunomodulatory properties against pathogenic microorganisms, as well as better control of infection by reducing bacterial counts.
Sepsis is a systemic inflammation associated with infection caused by pathogenic micro-organisms. Gram-negative and Gram-positive bacteria, viruses, and fungi can be responsible for the excessive stimulation of immune system and deregulate the protective response of the host.1 Sepsis remains a clinical problem in the XXI century. The increased incidence and mortality has evolved substantially over the past two decades, particularly in intensive care units.2,3 Despite the intense treatment to contain the infection in patients with sepsis, the use of drugs has not been satisfactory in clinical applications, resulting in a significant impact on health systems around the world, both economically and socially.4
The immunomodulation in sepsis has evolved in recent years, stimulated research centers in the investigation for therapeutic targets capable of containing the hyperactivity of the host immune system.5,6 The intervention immune is based on the pathophysiology of sepsis that causes the stimulation of intracellular signaling pathways leading to the gene expression of pro and anti-inflammatory, including TNF-α, IL-6, IL-10, and MCP-1.7,8 It has been proposed that the therapeutic management using immunomodulators is effective in infection because of its importance in the activation of cell populations and in the stimulation or suppression of immune mediators.9,10
Propionibacterium acne, formerly known as Corynebacterium parvum is a Gram-positive anaerobic micro-organism belonging to the skin human, being widely used in clinical trials as suitable candidate for therapeutic approach. The bacillus has been used as a tool for studies of cellular immune response due to its immunomodulatory effects.11 The inactivated bacteria promotes Th112 and Th213 response in mice, induces tumoricidal activity,14 increases resistance to viral infections,15 parasitic,16,17 has anti-bacterial activity18 and has an important relationship with the activation of cell populations.19,20 One of the most important adjuvant effects of P. acnes is the increase in macrophage phagocytic activity, which can explain, in part, by ability to enhance animal resistance to several pathogens.20
The immunomodulator P. acnes has been studied by several researchers, both in human and animal models of infectious diseases.21–24P. acnes action was evaluated in mice infected by the rabies virus and researchers observed greater survival rates in animals treated with the immunomodulator and antirabies vaccine. This greater survival was not related to serum neutralizing antibodies, interferon gamma concentration and cellular immunity evaluated by the macrophage inhibition test, but it was correlated with higher Natural Killer activity in infected mice treated using P. acnes.15
Although there are studies describing the immunomodulatory and antibacterial properties of P. acnes, we found no reports of its effect on polymicrobial sepsis. Thus, the objective of this study was to investigate the effects of P. acnes-Killed in lethal sepsis induced by cecal ligation and puncture (CLP).
MethodsExperimental animalsMale mice (Mus musculus) provided by the animal facilities of Federal University of Pernambuco – UFPE, Recife, Brazil, were used for the evaluation of the P. acnes-killed in sepsis model. The mice used weighed between 20 and 25g and were kept in a room with controlled temperature (22±2°C), humidity (50–60%) and a 12h/12h light/dark cycle. Water and food were made available to the animals without restriction. Before beginning the experiments, animals were acclimated to the laboratory environment for at least 30min. The Animal Studies Committee of the Federal University of Pernambuco approved the experimental protocols (number 23076.039440/2011-30). The animals were treated according to the ethical principles of animal experimentation of SBCAL (Brazilian Society of Laboratory Animal Science) and the norms of the National Institute of Health Guide for Care and Use of Laboratory Animals.
Drugs and reagentsP. acnes-killed was produced by Laboratório Farmacêutico do Estado de Pernambuco (LAFEPE), Brazil, constituted of 4mg/2mL of P. acnes-killed (marketed by name Imunoparvum®), dose used was 0.2mL (0.4mg)/animal. Other drugs and reagents used in this study were as follows: Agar Mueller Hinton (Difco Laboratories, USA). TNF-α, IL-6, IL-10, and MCP-1 kits were purchased from eBioscience, San Diego, California, USA.
Experimental designPolymicrobial sepsis was induced using CLP according to previously described method.25 After deep anesthesia, midline laparotomy was performed with exposure of cecum followed of ligature and one transverse perforation of the same, with a 18G needle, for induction of sepsis lethal. After surgery, the cecum of the animals was replaced into peritoneal cavity, and it was closed in two layers with a 4.0 nylon suture. Immediately after surgery, each animal received a subcutaneous injection of a 1mL of saline solution as fluid resuscitation.
The animals were initially divided into 3 groups (n=16) that were treated by intramuscular route in 1, 3, 5, and 7 days before the CLP induction. The control group animals (G-1) received vehicle (saline solution 0.9%) and the animals of the group 2 (G-2) received the P. acnes-killed constituted with 4mg/2mL of P. acnes-Killed at a dose of 0.2mL (0.4mg/animal). In the sham-operated group (G-3), underwent identical laparotomy but without cecum puncture and the mice were not treated. Six hours after CLP, half of the animals was euthanized to assess specific parameters (leukocytes count, determination of cytokines and bacteria counts in peritoneal fluid). The other half of each group was used for evaluation of the survival rate.
Cellular migrationSix hours after CLP, eight animals of each group were euthanized and the cells present in the peritoneal cavity were harvested by introducing 3mL of phosphate-buffered saline (PBS) containing 1μM of EDTA. Total white blood cells (WBC) count was performed by using an automatic counter (ABX Micros 60), and differential cell counts were carried out on cytocentrifuge slides (Cytospin 3; Shandon Southern Products, Astmoore, United Kingdom), stained by the panotic dye and analyzed by optical microscopy. The results were expressed as the number of cells per cavity.
Determination of cytokines in peritoneal lavageThe exudates collected from the peritoneal cavity were centrifuged and the supernatant stored in freezer at −20°C for the determination of cytokine levels. Quantification of the TNF-α, IL-6, IL-10, and MCP-1 levels in these exudates was performed by sandwich ELISA using kits that were specific for mice according to the manufacturer's instructions (eBioscience, San Diego, California, USA).
Determination of bacterial countsBacterial counts were performed on aseptically obtained peritoneal fluid. Six hours after CLP, mice were euthanized and the skin of abdomen was cut open in the midline without injury to the muscle. Samples were serially diluted from 1:100 to 1:1000 and 1:10,000 in PBS and cultured on Agar Mueller Hinton (Difco Laboratories) plates. Colony-forming units were counted after 18–24h incubating 32°C and the results were expressed as log10 of the number of colony-forming units/mL peritoneal fluid.
Statistical analysisThe results were expressed as the mean±standard deviation. Statistical analyses were performed using the Graph Pad software version 5.0 (GraphPad Software Inc., San Diego, CA, USA). We used a one-way ANOVA followed by a Bonferroni's test, with a significance level of 0.05%. The survival of mice was evaluated using curve-Mantel Cox and log-rank tests to compare the curves. The t-test was used for the comparison of data obtained for the enumeration of bacteria in the peritoneal cavity, with a significance level of 0.05%.
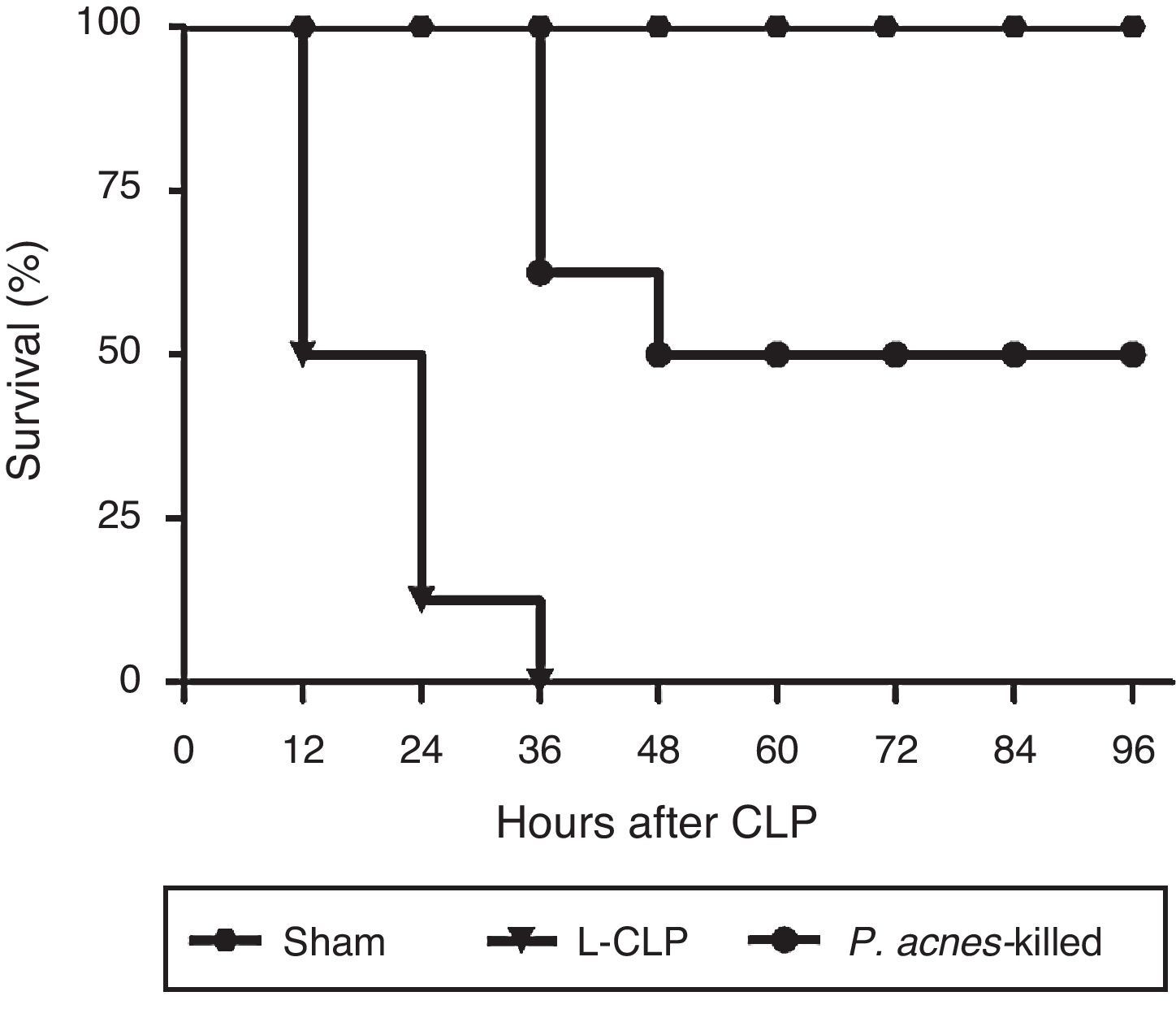
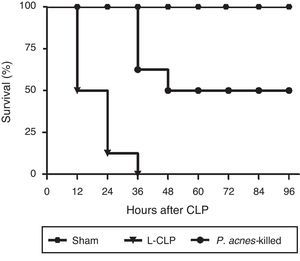
ResultsEffect of P. acnes-killed on the survival of animals subjected to lethal sepsis by CLPAs shown in Fig. 1, CLP induced the death of 100% of control animals 36h after surgery. The reduction in mortality was significant in the animals receiving treatment with P. acnes-killed. The rate of the treated group was 50% at 96h after CLP.
Effect of P. acnes-killed on survival rate of cecal ligation and puncture (CLP) rats (n=8). The data are expressed as the cumulative percentage of rats still alive within each interval and the survival each 6h was recorded. Compared with CLP group, P. acnes-killed improved survival within 96h. All animals survived in the sham group. p<0.05 vs CLP group.
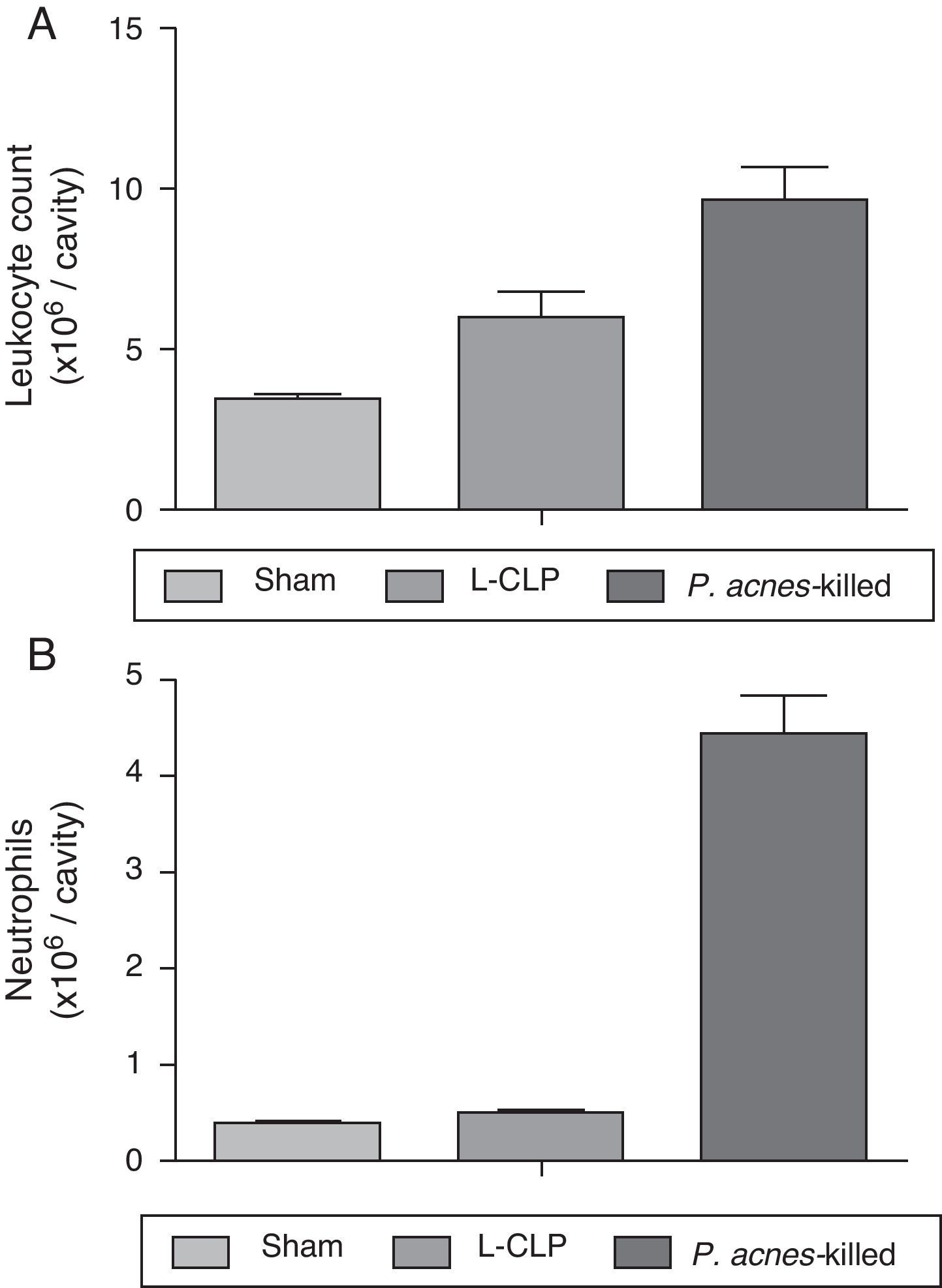
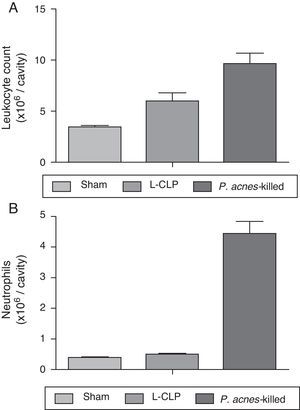
A significant increase in the total number of leukocytes were observed in the peritoneal cavity of the group treated with P. acnes-killed (9.64±2.7×106 leukocytes/cavity), in the control group (6.00±2.0×106 leukocytes/cavity) (Fig. 2). The recruitment of neutrophils into the peritoneal cavity increased in the group of animals that received P. acnes-killed compared with the control group.
Total (A) and differential (B) cell counting in the peritoneal cavity. The total and differential counting of peritoneal cells were performed 6h after the cecal ligation and puncture (CLP) (A). The results were expressed as mean ± S.E.M. (7 animals/group). *p<0.05 when compared to the control group.
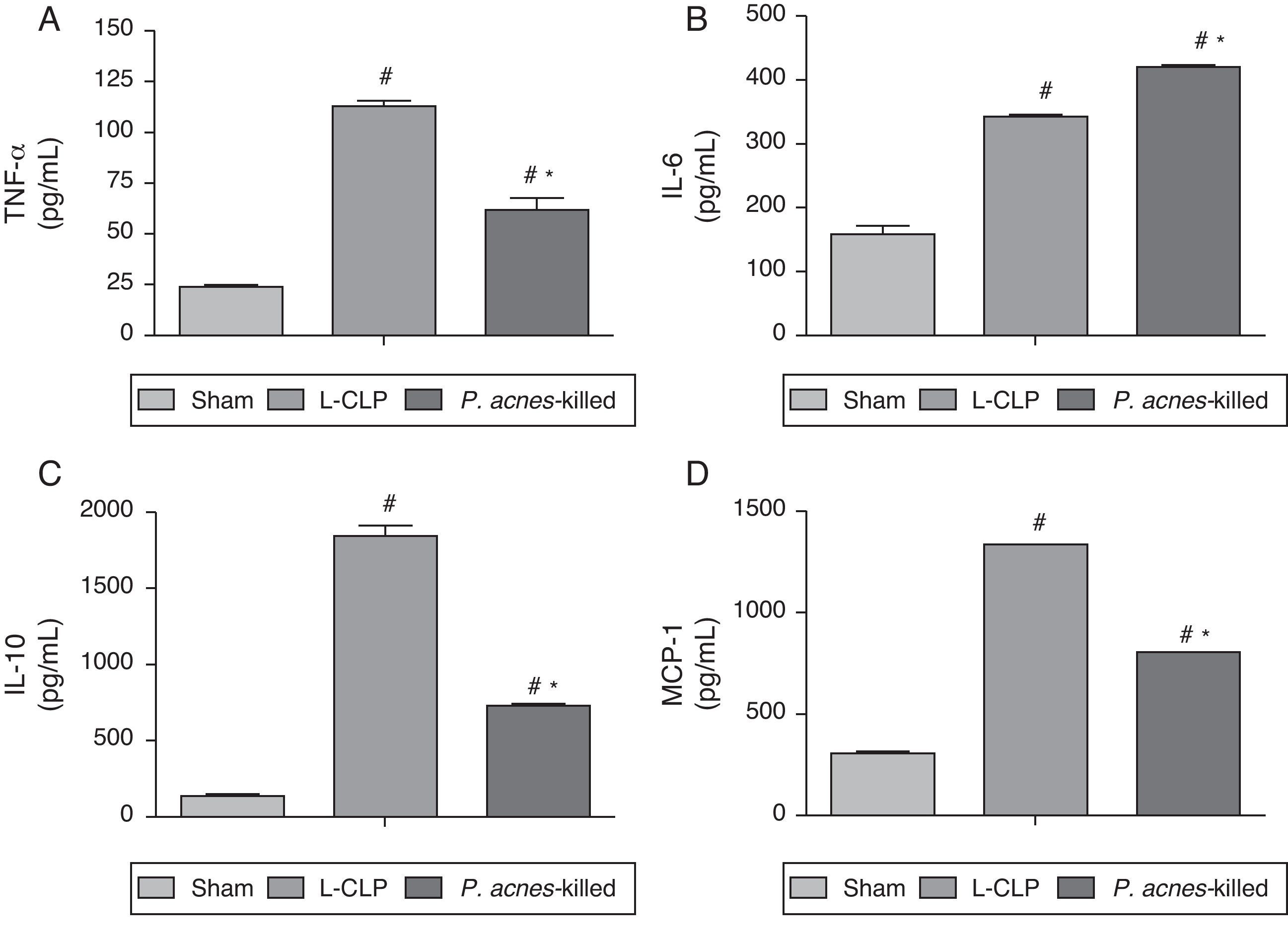
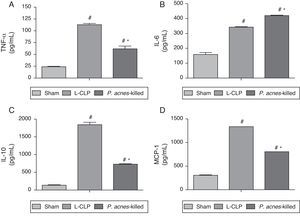
Fig. 3 shows the concentrations of TNF-α, IL-6, IL-10, and MCP-1 in the peritoneal exudate of the groups treated with P. acnes-killed, sham, and control group 6h after surgery. The levels of TNF-α, IL-10, and MCP-1 showed a decreased in animals treated with P. acnes when compared with the control group. In contrast, IL-6 increased through treatment with P. acnes-killed displayed high concentration 6h after CLP.
Effect of P. acnes-killed treatment in animals (n=6) in the expression of TNF-α (A), IL-6 (B), IL-10 (C) and MCP-1 (D) levels in the peritoneal cavity of mice subjected to cecal ligation and puncture (CLP). The cytokine levels in peritoneal exudates were determined at 6h after surgery in sham-operated, L-CLP and P. acnes-killed mice. Results are expressed as mean±S.E.M. *p<0.05 compared with L-CLP.
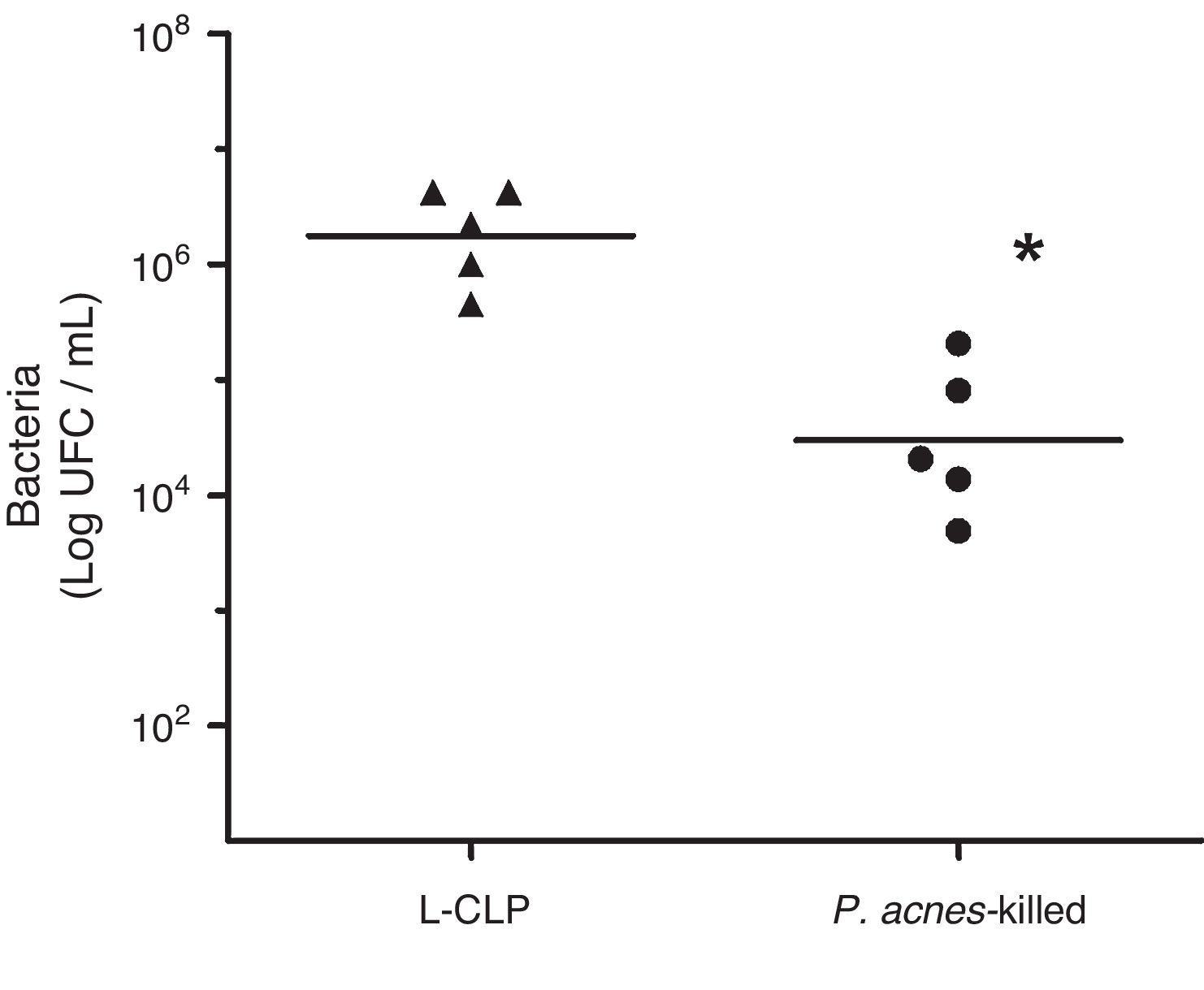
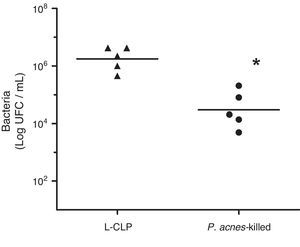
To evaluate the effect of P. acnes-killed in control of infection, we investigated the number of bacteria in the peritoneal cavity of animals subjected to lethal sepsis by CLP. We have detected that 6h after surgery the animals in the control group showed deficiency in control the infection, which was evidenced by the high concentration of colony-forming units (CFU) of the abdominal cavity. The number of bacteria found in the peritoneal cavity of the treated group was lower than the values found in control animals. The sham group had no CFU 24h after surgery (Fig. 4).
Bacterial counts in the peritoneal fluid of L-cecal ligation and puncture (CLP) and Imunoparvum® groups (n=5). Quantification of the amount of bacteria in the peritoneal cavity was performed 6h CLP surgery. The number of bacteria present in the peritoneal cavity is expressed as mean logCFU/mL. **p<0.05 compared with L-CLP.
This study demonstrated that the protective action of P. acnes-killed modulates the inflammatory response in animals subjected to lethal sepsis by CLP. When the animals received an intramuscular injection of P. acnes-killed and underwent severe sepsis there was a reduction in mortality, conferring resistance to infection. In this study we have observed a survival rate of 100% in the first 24h after surgery in the treated group. CLP is a murine model of polymicrobial sepsis similar to the progression and characteristics of human sepsis that has contributed to the knowledge of the involvement of components of innate immunity, including the identification of new potential therapeutic targets.26 The sensitivity of animals treated with P. acnes is not restricted to Gram-negative bacteria by LPS, but also to Gram-positive bacteria such as Listeria monocytogenes.27 The data found in our study are in agreement with other authors who reported the antibacterial activity of P. acnes against Gram-positive and Gram-negative bacteria.28,29
In the sepsis, a failure of neutrophil migration to the infectious site has been found and is associated with a large deficit in the control of infection, leading to increased bacterial dissemination and inducing a high mortality rate.30 The neutrophil migration to the site of infection is extremely important for the control of bacterial growth and consequently for the prevention of bacterial dissemination.31 This study demonstrated that the increase in animal survival was accompanied by an increase in migration of cells into the peritoneal cavity and significant decreased in colony forming units of the group treated with P. acnes-killed. Studies have shown that P. acnes has worked in the modulation of macrophages, eosinophils,13 neutrophils,32 dendritic cells,33 and natural killer cells.34 Our data corroborate the findings of other authors that observed the recruitment of neutrophils into the peritoneal cavity when animals were stimulated with P. acnes-killed.35
The expression of inflammatory mediators is the host response to infection. In sepsis, the over expression of proinflammatory cytokines have deleterious effects on the body, disrupt the immune system and is involved in multiple organ dysfunction.36 TNF-α is one of the mediators responsible for organ dysfunction and increased mortality during severe sepsis.37 Some studies have reported that increasing the survival rate is following the decrease of TNF-α.5,6 Thus, immunotherapy focus of sepsis in blocking TNF-α is promising in animal models. This study assessed the effect of P. acnes-killed on the concentration of TNF-α in the peritoneal cavity 6h after CLP and there was a significant reduction in levels of this marker. Similar results were found in peritoneal fluid by Pang et al.38 that evaluated the modulating effect of the methylene chloride in animals with sepsis and reported a decrease in TNF-α and consequent increase in survival. Another study found that the decrease in concentration of TNF-α in serum and peritoneal exudate of animals treated with myrrh, an essential oil obtained from species of the genus Commiphora, increased survival in severe sepsis.39 Recently it was shown the immunomodulatory activity of a polysaccharide of P. acnes on reducing TNF-α in animals with focal segmental glomerulosclerosis.40
The monocyte chemotactic protein (MCP-1) is a CC chemokine belonging to the subfamily that stimulates the migration of monocytes cells and is associated with protection in experimental sepsis with activation of neutrophils and macrophages.41 The coordinated synthesis and release of MCP-1 is important in acute and chronic inflammatory process by controlling the influx of phagocytic cells and also activation of the release of inflammatory cytokines.42 In our study there was a decrease in levels of MCP-1 in animals treated with P. acnes-killed and subjected to severe sepsis. Previous study shown that blocking the activity of MCP-1 in animals sensitized with P. acnes inactivated decreased of the concentrations of TNF-α and IL-β.43 Recently, anti-MCP-1 strategies have been suggested as a potential therapeutic for the treatment of sepsis and endotoxemia by preventing the interaction between MCP-1 and other inflammatory mediators and oxidative stress in organs.44
In this study, the data showed no decrease in the concentration of IL-6 following treatment with P. acnes-killed. The studies that evaluated the effects of P. acnes on the concentration of IL-6 are controversial. The increased IL-6 levels after stimulation with P. acnes has been reported,45 moreover, P. acnes also decreases IL-6 concentrations.46 Mice deficient for IL-6 showed no improvement in survival.47 In addition to administration of high doses of anti-IL-6 or lower doses of antibody did not increase survival of animals with sepsis. This suggests that excessive levels of IL-6 are detrimental and that the lock does not help the survival of these animals.48 Although P. acnes modulates the expression of IL-6 increasing its concentration in this experimental model, we believe that the presence of a balanced concentration of IL-6 in acute inflammation may be required for an effective immune response in innate immunity. Therefore, further analyses are needed to clarify this issue.
Notably, pre-treatment of P. acnes-killed suppressed the production of IL-10 in the peritoneal cavity. The cytokine IL-10 appears to be crucial in regulating the production of pro-inflammatory cytokines.49 In a previous study, administration of IL-10 reduced the levels of TNF-α and increased survival of endotoxemic animals.50 However, this was not the case of our study, because the treatment with P. acnes-killed reduced IL-10, but also decreased TNF-α and MCP-1. The role of IL-10 in polymicrobial sepsis or its interaction with pro-inflammatory cytokines may therefore be more complex. The decreased expression of IL-10 is associated with increased survival by restoring the host immune response51 and the decrease of CFUs in animals subjected to CLP.52 Recently it was suggested that neutralization of IL-10 decreases the percentage of Treg CD4+ T cells and increases the survival of septic animals.53 Thus, it is suggested that the reduced levels of IL-10 in this study was beneficial in protecting against polymicrobial sepsis.
Finally the data obtained in this study show that the beneficial effect of treatment with P. acnes-killed in the survival rate of animals was accompanied by reduced levels of TNF-α, MCP-1, and IL-10 and increased IL-6, contributing to leukocyte recruitment and reduced bacterial growth. These data suggest that the improvement of host defense in the initial focus of infection can weaken the inflammatory response in other places.
Conflict of interestThe authors have no conflict of interest.
The authors acknowledge financial support by CAPES and CNPQ.