Over the past several years, the epidemiological profile of dengue has been changing progressively and is currently characterized by an increase in the number of cases in children under 15 years of age. This study was aimed at comparing the clinical and laboratory features between adults and children with dengue; therefore, we performed a cross-sectional analysis of 5686 individuals with laboratory-confirmed dengue who sought treatment at a healthcare facility in Rio de Janeiro, Brazil from 2010 to 2011. A multivariate analysis indicated that myalgia (OR=2.58; CI 95%=2.08–3.18), retro-orbital pain (OR=1.36; CI 95%=1.15–1.62), nausea (OR=1.92; CI 95%=1.60–2.30), and arthralgia (OR=3.64; CI 95%=2.72–4.89) were the most frequent clinical symptoms in adults, whereas vomiting (OR=0.52; CI 95%=0.43–0.61) and skin rash (OR=0.46; CI 95%=0.25–0.85) were the most prevalent symptoms in children. Adults exhibited a higher hemoconcentration (OR=3.04; CI 95%=2.53–3.65), thrombocytopenia (OR=2.17; CI 95%=1.80–2.60), increased erythrocyte sedimentation rate (OR=1.81; CI 95%=1.53–2.14), and increased ALT (OR=3.13; CI 95%=2.44–4.02) than did children. In addition, adults exhibited a higher frequency of the severe forms of the disease (OR=1.74; CI 95%=1.12–2.72) and hospitalization (OR=2.21; CI 95%=1.59–3.06) relative to children. Based on these results, this study demonstrated significant differences in the clinical and laboratory presentations and disease severity between adults and children affected by dengue.
Dengue is an acute febrile disease. The etiologic agent of dengue is the dengue virus (DENV), which belongs to the Flaviviridae family and the Flavivirus genus and has four serotypes. Dengue is the most common arthropod-borne viral disease and is one of the most significant diseases because it is associated with high rates of morbidity and mortality.1–3
This disease currently represents a major public health issue, especially in tropical and subtropical countries, where environmental conditions enable the development and proliferation of the mosquito Aedes aegypti, which is the main vector of DENV.4,5
Dengue epidemiology has been changing in Brazil, with a shift being observed in the age group of the affected population. Currently, at least 25% of notified dengue cases occur in children who are 15 years of age or younger.6,7
The clinical manifestations of dengue vary from a nonspecific febrile disease to a more severe form with bleeding, thrombocytopenia, and plasma extravasation, which can result in death. In children, dengue can be asymptomatic or polysymptomatic, which may hinder the differential diagnosis.8,9
An early clinical diagnosis of dengue hemorrhagic fever is difficult because the criteria that were established by the World Health Organization (WHO) may be present only in the initial phase of the acute form of the disease.4 Thus, laboratory tests combined with a guided clinical examination, especially in endemic areas, are crucial to confirm a diagnosis, identify, and prevent outbreaks and provide early treatment for the severe forms of the disease and complications.9
There are few reports on the clinical and laboratory differences in children (0–14 years of age) and adults with dengue.10 Thus, this study aimed to determine the differences among individuals with dengue who were admitted to the Reference Center for Dengue (Centro de Referência da Dengue, CRD) in Campos dos Goytacazes, Rio de Janeiro.
Material and methodsStudy design and subjectsThis study was a cross-sectional, retrospective analysis that was conducted at the Reference Center for Dengue (Centro de Referência da Dengue, CRD), which is located at Campos dos Goytacazes city, Rio de Janeiro, Brazil. The medical records of all of the individuals who were admitted to the CRD from January 2010 to September 2011 were evaluated. The CRD Evaluation Protocol was used as a tool for data collection, which consists of a questionnaire on the epidemiological, clinical, and laboratory data that were collected during the first medical visit. Individuals with a dengue diagnosis that was based on clinical and serological criteria were included in the study.
DefinitionsSuspected cases of dengue were defined as individuals who were living in or traveled to dengue-endemic areas within the previous 15 days and who presented with fever combined with at least two of the following symptoms: nausea, vomiting, skin rash, myalgia, arthralgia, positive tourniquet test, leucopenia, and any other warning sign. The latter symptoms of suspected cases included the following: pain or abdominal discomfort, persistent vomiting, serositis, mucosal bleeding, lethargy, agitation, hepatomegaly >2cm, and an increase in the hematocrit with a simultaneous decrease in platelets.11
Following the current WHO guidelines, suspected cases were classified as severe and nonsevere. Severe cases were defined as the following: severe plasma extravasation that led to dengue shock syndrome (DSS) or liquid accumulation with respiratory discomfort; severe hemorrhage that was evaluated by the physician; severe commitment of tissues, such as ≥1000 aminotransferases; and commitment of the central nervous system, heart, and other organs.11
Laboratory methodsIn the first medical visit, individuals who presented with the clinical and epidemiological indications for dengue were referred to the laboratory for peripheral blood collection and the following laboratory tests: complete blood count (CBC), erythrocyte sedimentation rate (ESR), aspartate aminotransferase (AST), and alanine aminotransferase (ALT). To minimize bias, the tests were performed at a laboratory that was associated with the CRD. The hematocrit (Ht) parameters that were used to evaluate hemoconcentration included an adult man with a Ht>45%, an adult woman with a Ht>40%, and children with a Ht>38%. Leukopenia was defined as a leukocyte count of <4000/μL, and thrombocytopenia was defined as a platelet count of <150,000/μL. An ESR result >20mm/h, an AST result >50U/L, and an ALT result >50U/L were considered to be high.
All of the dengue cases were confirmed in the laboratory by the following: (1) serological tests using an immunoenzyme assay (PANBIO® Indirect ELISA for anti-DENV IgM) for the capture of anti-dengue IgM with blood that was collected between days 5–10 after the first fever symptoms; and (2) detection of the NS1 antigen of DENV using an immunoenzyme assay (BIORAD® Platelia Dengue NS1 Assay™) with samples that were collected between days 1–4 after the first fever symptoms.
TreatmentSevere cases were admitted to the CRD support hospital (Hospital dos Plantadores de Cana) to receive proper clinical and laboratory treatment. The nonsevere cases with no alarming clinical signs were admitted to the CRD ambulatory clinic. The nonsevere cases who presented with alarming clinical signs were treated individually, and clinical support was provided at either the hospital or ambulatory clinic according to the severity of each case.
Statistical analysisA statistical analysis was performed to evaluate the differences in the clinical and laboratory manifestations among individuals with dengue from the two age groups: adults and children. For the univariate analysis, a chi-square analysis or Fisher's exact test were performed for the categorical variables. Variables with a p-value lower than 0.10 were used for a multivariate logistic regression model. p-Values lower than 0.05 were considered to be significant. A data analysis was performed with the software program SPSS 13.0 for Windows (Statistical Package for the Social Sciences, Chicago, IL, USA).
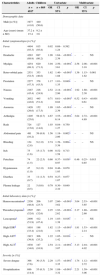
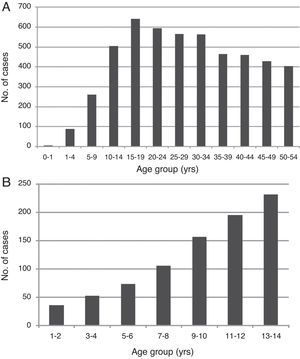
ResultsA total of 5686 individuals with confirmed dengue were evaluated: 4817 were adults (84.7%), and 869 were children (15.2%). The majority of adults were female, who represented 59.0% (2840) of adults and 49.4% (429) of children. The average adult age was 37.2 years, and the average child age was 9.2 years (Table 1). Adults who were 15–19 years of age and children who were 10–14 years of age were the most affected groups (Fig. 1).
Univariate and multivariate comparative analysis of initial symptoms/signs, laboratory data and dengue severity in adults and children with dengue.
| Characteristics | Adults | Children | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|---|---|
| n=4817 | n=869 | OR | CI 95% | p | OR | CI 95% | p | |
| Demographic data | ||||||||
| Male [n (%)] | 1977 (41.0) | 440 (50.6) | ||||||
| Age (years) (mean±SD) | 37.2±15.4 | 9.2±3.6 | ||||||
| Initial symptoms/signs [n (%)] | ||||||||
| Fever | 4494 (93.3) | 815 (93.8) | 0.92 | 0.69–1.24 | 0.592 | |||
| Headache | 4283 (88.9) | 697 (80.2) | 1.98 | 1.64–2.39 | <0.001* | – | – | NS |
| Myalgia | 4254 (88.3) | 620 (71.3) | 3.04 | 2.56–3.61 | <0.001* | 2.58 | 2.08–3.18 | <0.001 |
| Retro-orbital pain | 2531 (52.5) | 353 (40.6) | 1.62 | 1.40–1.88 | <0.001* | 1.36 | 1.15–1.62 | 0.001 |
| Prostation | 2277 (47.3) | 378 (43.5) | 1.17 | 1.01–1.35 | 0.040* | – | – | NS |
| Nausea | 2187 (45.4) | 216 (24.9) | 2.52 | 2.14–2.96 | <0.001* | 1.92 | 1.60–2.30 | <0.001 |
| Vomiting | 2052 (42.6) | 445 (51.2) | 0.71 | 0.61–0.82 | <0.001* | 0.52 | 0.43–0.61 | <0.001 |
| Anorexia | 1428 (29.6) | 152 (17.5) | 1.99 | 1.65–2.39 | <0.001* | – | – | NS |
| Arthralgia | 1205 (25.0) | 58 (6.7) | 4.67 | 3.55–6.14 | <0.001* | 3.64 | 2.72–4.89 | <0.001 |
| Rash | 724 (15.0) | 127 (14.6) | 1.03 | 0.84–1.27 | 0.750 | |||
| Abdominal pain | 491 (10.2) | 59 (6.8) | 1.56 | 1.18–2.06 | 0.002* | – | – | NS |
| Bleeding | 134 (2.8) | 9 (1.0) | 2.74 | 1.39–5.39 | 0.004* | – | – | NS |
| Itch | 75 (1.6) | 15 (1.7) | 0.90 | 0.52–1.58 | 0.713 | |||
| Petechiae | 74 (1.5) | 22 (2.5) | 0.60 | 0.37–0.97 | 0.038* | 0.46 | 0.25–0.85 | 0.013 |
| Hypotension | 47 (1.0) | 9 (1.0) | 0.94 | 0.46–1.93 | 0.870 | |||
| Diarrhea | 33 (0.7) | 11 (1.3) | 0.54 | 0.27–1.07 | 0.077 | |||
| Plasma leakage | 22 (0.5) | 5 (0.6) | 0.79 | 0.30–2.10 | 0.640 | |||
| Initial laboratory data [n (%)] | ||||||||
| Hemoconcentrationa | 2350 (48.8) | 206 (23.7) | 3.07 | 2.60–3.62 | <0.001* | 3.04 | 2.53–3.65 | <0.001 |
| Thrombocytopeniab | 2507 (52.0) | 283 (32.6) | 2.25 | 1.93–2.62 | <0.001* | 2.17 | 1.80–2.60 | <0.001 |
| Leucopeniac | 2498 (51.9) | 412 (47.4) | 1.19 | 1.03–1.38 | 0.016* | – | – | NS |
| High ESRd | 1610 (33.4) | 188 (21.6) | 1.82 | 1.15–1.54 | <0.001* | 1.81 | 1.53–2.14 | <0.001 |
| High ASTe | 1915 (39.8) | 306 (35.2) | 1.22 | 1.05–1.42 | 0.010* | – | – | NS |
| High ALTf | 1638 (34.0) | 147 (16.9) | 2.54 | 2.11–3.07 | <0.001* | 3.13 | 2.44–4.02 | <0.001 |
| Severity [n (%)] | ||||||||
| Severe dengue | 366 (7.6) | 30 (3.5) | 2.29 | 1.57–3.35 | <0.001* | 1.74 | 1.12–2.72 | <0.001 |
| Hospitalization | 696 (14.4) | 55 (6.3) | 2.50 | 1.88–3.32 | <0.001* | 2.21 | 1.59–3.06 | <0.001 |
OR, odds ratio; CI 95%, 95% confidence interval; ESR, erythrocyte sedimentation rate; AST, aspartate aminotransferase; ALT, alanine aminotransferase; DHF, dengue hemorrhagic fever; NS, not significant.
Regarding the signs and symptoms that were identified in the first evaluation at the CRD, classical signs, such as fever, headache, and myalgia, were present in over 70% of adults and children with dengue (Table 1). Common clinical manifestations, such as retro-orbital pain, prostration, nausea, and vomiting, were observed in 40–50% of adults and children.
A comparison among age groups revealed that the frequency of clinical signs and symptoms was significantly different between adults and children. The univariate analysis indicated that headache, myalgia, anorexia, retro-orbital pain, abdominal pain, nausea, arthralgia, prostration, and bleeding were present more frequently in adults than in children. However, the multivariate analysis indicated that only myalgia (OR=2.58; CI 95%=2.08–3.18), retro-orbital pain (OR=1.36; CI 95%=1.15–1.62), nausea (OR=1.92; CI 95%=1.60–2.30), and arthralgia (OR=3.64; CI 95%=2.72–4.89) were significantly associated with adults with dengue. Vomiting (OR=0.52; CI 95%=0.43–0.61) and rash (OR=0.46; CI 95%=0.25–0.85) were the correlated symptoms in children in both the univariate and multivariate analyses.
Regarding the laboratory evaluations, all of the alterations (hemoconcentration, thrombocytopenia, leukopenia, and increases in ESR, AST and ALT) were more frequent in adults than in children. However, the multivariate analysis demonstrated significant differences between adults and children only for the hemoconcentration (OR=3.04; CI 95%=2.53–3.65), thrombocytopenia (OR=2.17; CI 95%=1.80–2.60), increased ESR (OR=1.81; CI 95%=1.53–2.14), and increased ALT (OR=3.13; CI 95%=2.44–4.02) (Table 1).
Adults had a higher risk for severe dengue (7.6% vs. 3.5%) and a higher frequency of hospitalization (14.4% vs. 6.3%) than did children (Table 1). The differences were significant in both the univariate and multivariate analyses for severe dengue (OR=1.74; CI 95%=1.12–2.72) and hospitalization (OR=2.21; CI 95%=1.59–3.06) (Table 1).
DiscussionIn this study, we detected a higher prevalence of women who were infected with dengue than of men. These data are similar to those reported by a study in Nicaragua3 and different from other studies.10,13,14,15 We did not observe a significant difference in gender when it was correlated with the age groups.
The children with age between 10 and 14 years old and adults between 15 and 19 years old were the age groups most prevalent affected, similar to the data reported previously.13
In contrast to some studies,3 we found a higher prevalence of headache, myalgia, arthralgia, retro-orbital pain in adults. These findings may be related to the difficulty in identifying these signs and symptoms by the parents and the children. Abdominal pain is also more common in adults, maybe because of the anatomo-physiological differences of the organs affected by dengue according to age.10
There were more hemorrhagic manifestations in adults. It is believed that the primary infection acts as a protective factor against the hemorrhagic forms in children since repeated infections by different serotypes of the DENV, results in more severe manifestations.10
We found a higher prevalence of petechiae and vomiting in children, as opposed to other studies.12
As reported in the literature, on Dengue's Fever, leukopenia and thrombocytopenia were the most prevalent laboratory findings.10,16,17 The induced viral destruction or the inhibition of myeloid progenitor cells causes leukopenia and peripheral destruction of platelets or destruction of megakaryocytes of the bone marrow by the virus, resulting in decreased production of platelets. There were no clinical correlation between thrombocytopenia and severity of Dengue.16
The literature on dengue infection indicates that an increase in aminotransferases is a relevant laboratory result.1,16,18 In this study, we observed that increased ALT and AST levels were frequent; however, there was no correlation between these results and the severe forms of dengue.16,19
The laboratories alterations such as thrombocytopenia and elevation of ALT were more severe in adults, like published before.10 It is noteworthy that we also did not find respiratory and renal involvement in any of the cases studied.
Several studies have suggested that adults who have been previously primed with DENV have a favorable prognosis; however, subsequent infections by distinct DENV serotypes may result in severe forms of the disease. Primary infection could be a protective factor against the severe forms of dengue in children, which may explain the higher incidence of the severe forms of the disease and hospitalization in adults.10
In this study, a low prevalence of severe cases (7.0%) and hospitalization (13.2%) was observed. We ascribe these findings to the efficacy of the CRD Protocol, which advises patients to seek medical assistance immediately after the onset of clinical manifestations and recommends that a diagnosis be based on the clinical evolution of the disease, thereby reducing the risk of disease development to the severe forms, such as DSS.20
According to the Notifiable Diseases Information System (Sistema de Informação de Agravos de Notificação – SINAN) of the Ministry of Health of Brazil, Campos dos Goytacazes (where CRD is located) has the lowest mortality rate (1.3%) in Rio de Janeiro, whereas the capital has a rate of 4.8%, and other cities may reach a mortality rate of 10% among severe cases. These findings reinforce the need to standardize programs for the early diagnosis and treatment of dengue, such as the programs conducted at the CRD.
ConclusionThe present study detected the presence of classic signs and symptoms (fever, headache and myalgia) in 70% of the adults and the children with this dengue. Many signs and symptoms of dengue manifest differently in adults and children. Adult patients with dengue typically present symptoms of myalgia, retro-orbital pain, nausea and arthralgia. Children manifests mainly with vomiting and petechiae. There were no differences between these two age groups about the presence of fever, rash, itch, hypotension and diarrhea. Hemoconcentration, thrombocytopenia, ALT and ESR elevation occurred predominantly among adults. Adult patients also had a higher risk of severe dengue and an increased frequency of hospitalization.
Authors’ contributionsLCM, LAS, MBTR, LBP, MVS, LJS and JTDSF were involved with conception and design of the study. LJS coordinated the study. LCM, LAS, MBTR, LBP and MVS collected clinical and epidemiological data. JTDSF did the analysis and interpret the data. LCM, LAS, MBTR, LBP, MVS, and JTDSF wrote the manuscript. All authors reviewed the manuscript critically, improved the intellectual content, and approved the final version. LJS is the guarantor of this paper.
Conflict of interestThe authors have no conflict of interest to declare.
Ethical approvalThis study was conducted in accordance with the Declaration of Helsinki, the International Conference on Harmonisation Good Clinical Practice Guidelines and all relevant Brazilian laws and regulations.
FundingThis study was funded in part by Brazilian Society of Internal Medicine/Rio de Janeiro State.
The authors thank Paulo Roberto Hirano and Charbell Miguel Haddad Kury (Secretaria Municipal de Saúde de Campos dos Goytacazes, Campos dos Goytacazes-RJ) for collaboration and incentive of this research.