Human papillomavirus (HPV) has been found in several regions of the body, including the oral cavity. Recently, this virus has been associated with oropharyngeal cancer, but little is known about HPV transmission to the oral cavity. We carried out a study to investigate concurrent oral and cervical infections in 76 asymptomatic women attending a healthcare program. Demographic and behavior data were obtained through a structured questionnaire. Oral and cervical mucosa scrapings were collected and stored for DNA extraction. HPV DNA amplification was performed by polymerase chain reaction assay (PCR) using both primers My09/My11 and FAP59/64, followed by HPV typing with restriction fragment length polymorphism analysis (RFLP) and sequencing. The data collected revealed no risk factors for HPV infection in these 76 women. HPV prevalence of 9.2 and 5.3% was found in cervical and oral mucosa, respectively. Concurrent infections by discordant types were detected in one case only. Sequencing procedures allowed us to detect a new putative HPV 17 subtype from the Betapapillomavirus genus. Our results support the view that cervical and oral HPV infections are independent events. The observed low prevalence of both oral and cervical HPV infections could be associated with attendance in a healthcare program.
Human papillomavirus species from Alpha, Beta, and Gamma genera have been found in oral cavities, but their transmission mechanism remains unclear. Acquisition could be through oral–genital or mouth-to-mouth contact, autoinoculation, or an independent event.1 This matter is controversial, and Rautava and Syrjänen2 concluded that oral infection can be transmitted sexually, through kissing or by household contact, or even by vertical transmission from mother to child during childbirth. Vidotti et al.3 reported a significant association between oral and genital HPV. On the other hand, genital infection could not predispose to oral infection, even if both sexual partners practiced oral sex.4 Oral HPV infections are frequently asymptomatic, but in the last decade some types were also associated with oropharyngeal cancer.5
Currently, three anti-HPV vaccines are commercially available. Although clinical trials have shown high efficacy in the prevention of malignancies in uninfected women, the impact of these vaccines on non-genital infections have not been determined yet.6 However, Herrero et al.7 stated that a bivalent vaccine is effective in preventing oropharyngeal cancer. If vaccination is effective for some HPV types of the genital area, it is quite possible that it also acts in other parts of the body. The investigation of HPV genotypes in the cervical and oral tract of asymptomatic women who regularly attended a health program may contribute to the knowledge of oral HPV transmission.
We conducted a randomized, cross-sectional survey, initially investigating 351 sexually active women who were referred to the Family Health Program in Niterói, Rio de Janeiro State, from 2009 to 2010. Data about this study have been published elsewhere.8
Out of the 351 participants, 84 asymptomatic women without oral lesions agreed to participate in oral HPV tests. After undergoing a routine gynecological examination, two cervical specimens were collected from each participant for Papanicolaou staining and HPV testing. None of the women had been vaccinated against HPV. A scraping of the oral cavity from the borders of the tongue and cheeks was also performed using a sterile brush. Demographic data were obtained through a structured questionnaire. The Ethics Committee of the College of Medicine of the University provided ethical clearance for the protocol and informed consent (CEP CMM/HUAP 052/2010, CAAE 0037.0.258.000-10).
DNA was extracted from samples (High Pure PCR Template Preparation Kit, Roche, São Paulo, Brazil) and quantified (Thermo Scientific NanoDrop 2000 spectrophotometer, Wilmington, North Carolina, USA). Due to insufficient DNA, eight scrapings were discarded, remaining 76 samples. The quality of DNA extracted was assessed by polymerase chain reaction assay (PCR) amplification of a 260bp DNA fragment for β-globin using the primers PC04/GH20.9 Amplification of HPV DNA was done with MY09/11 consensus primers (Síntese Biotecnologia, Belo Horizonte, MG), which amplify a 450bp in the L1 region of HPV DNA.10 To improve the sensitivity of the assay to detect oral HPV, a second PCR system using the primers FAP59/6411 (Síntese Biotecnologia, Belo Horizonte, MG) was added. These primers amplify a 480bp within the same L1. All oral samples were submitted to My09/11 and FAP/64 systems. Although MY09/11 degenerate primers are routinely and largely used to detect mucosal HPV (Alpha species), especially from genital mucosa, the FAP 59/64 system has been applied in the detection of cutaneous HPV (Beta and Gamma species) and has been used to improve detection of oral HPV.12 The combination of these systems tracks a broad range of types, even types not yet identified.
HPV typing was performed by restriction fragment length polymorphism analysis (RFLP) or sequencing. For RFLP analysis, the resultant 450 base pairs PCR products were digested by six restriction enzymes (BamHI, DdeI, HaeIII, HinfI, PstI, RsaI; Invitrogen, São Paulo, Brazil). The RFLP pattern of each sample was analyzed by agar gel electrophoresis under ultraviolet light and compared with the RFLP patterns for mucosal HPV.13 The smears that could not be typified by RFLP assay were submitted to sequencing. The amplified PCR product was purified and directly sequenced in an ABI DNA 3130 sequencer (Applied Biosystems, California, USA) with the same primers as those used for PCR amplification. The forward and reverse sequences were aligned and analyzed using ClustalW in BioEdit Sequence Alignment Editor (North Carolina State University, Raleigh, NC).14 Similarity analysis was performed using BLAST (Alignment Search Tool Analysis).15 Types that could not be classified by any methods were considered to be unidentified. A databank was generated in the SPSS-18 statistical package. Chi-Square (χ2) or Fisher's exact test were applied to compare categorical variables. t-Tests were used for comparing age means among groups. To identify associations between possible risk factors and the presence of HPV, odds ratios (OR) with 95% confidence intervals (95% CI) were calculated. For all tests, the level of significance was set at 0.05.
The participants who enrolled in this study were aged 14 through 70 years, with a mean age of 37.26 years and standard deviation of 14.59; 60.5% of the women were over 30 years old. Most of them received a monthly family income of over U$ 600, and 63.2% had attended only elementary school or reported being illiterate. Non-white people were the most prevalent ethnic group (69.7%). Most of the women maintained a stable sexual partner and had up to three lifetime sexual partners, with one partner during the last three months. Nearly half had begun their sexual activity before the age of 17. Sixty-six percent and 52.50% of the women had never used cigarettes or alcohol, respectively. Condoms were not used by 78.9%, but other contraceptive methods were employed by 84.2% of them. Parity up to two children was reported by 69.2% of the women, of whom 32.8% reported abortion episodes.
At the time of the interview, most of the participants (81.6%) had no history of any sexually transmitted disease. The remaining people reported past vulvar condyloma (7), syphilis seropositivity (4), candidiasis (2), and chlamydia (1). No women reported being HIV positive. Most of these women were submitted to regular gynecological examinations (93.4%). The cervical oncologic cytology smear from 94.7% of the participants was normal or inflammatory. The results show that a predominantly healthy population concerning cervical lesions, risk factors for HPV infection, and sexually transmitted diseases was screened. Therefore, educational support from basic healthcare staff could be associated with the profile of this population.
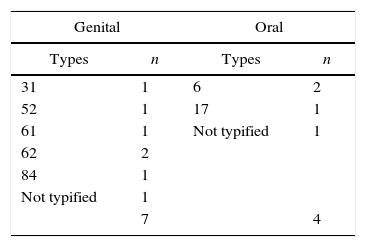
Genital and oral HPV were present in 9.2 and 5.3% of the women, respectively. The cervical and oral types are shown in Table 1. Each site harbored types totally discordant and the only woman HPV positive for both sites harbored different HPV types. In line with our findings, a German research reported that HPV oral autoinoculation and transmission to sexual partners were uncommon events in 60 cervical HPV-positive women.16 Similarly, Kero et al.17 concluded that although oral and genital HPV infections are common in current partners, the low concordance of types supports the hypothesis that the current HPV types had been established long before the current relationship. Our results support this statement.
The high-risk type 16, frequently detected in both sites, was missing in this population. We also had not found HPV 16 in a previous research on oral HPV detection enrolling 77 HIV-infected people and 120 healthy controls.18 Other authors have described the low or rare prevalence of HPV 16 in the oral cavity.19,20 In a Brazilian study, HPV genotypes 6 and 11 were the types most commonly detected in the oral mucosa. Type 16 was only found in one sample (Cordeiro TI, not published). HPV 61, 62, and 84, tracked in this study, are infrequent and probably transient. Interestingly, only one out of seven women with past condyloma harbored genital HPV infection (type 62), confirming the self-limited nature of these lesions as well as the transient character of low risk HPV associated with them. In the vaccination era, such information can be helpful.
Despite the sensitivity of the methods used for viral detection, which may be affected by factors such as DNA concentration and purity, the combination of two primer sets was successful. We obtained 10 infected samples using the primers My09/11, seven of which were positive for cervical HPV and three positive for oral HPV. The three positive samples for MY PCR were also positive for FAP/64 PCR. One of them, which could not to be identified by RFLP, was typified by FAP/64 PCR followed by sequencing as type 6. One sample was exclusively detected by FAP/64 PCR, and the sequencing revealed an unidentified type genetically close to the genus Betapapillomavirus.
We detected a new putative HPV 17 subtype in the oral cavity using the primers My09/11. The partial analysis of the L1 DNA sequence showed a genome not very close to the prototype. Although two subtypes (“a” and “b”) have been described for HPV 17, the alignment of these regions with known sequences showed a dissimilarity higher than 2%. Isolates difference between ≥90% and ≤98% identity in the partial L1 sequence are considered subtypes, but the final result will be defined by the total sequence.
In conclusion, our results suggest that oral HPV infection is unlikely to be acquired through autoinoculation. Oral HPV infection is rather an independent event from cervical infection, strengthening the hypothesis of different transmission routes. The low prevalence of both infections was probably linked to compliance with guideline recommendations regarding practices to avoid sexually transmitted diseases. Therefore, we hope that HPV vaccination does not compromise cervical cancer control screening programs. The finding of a new putative oral HPV 17 subtype underscores the large spectrum of viral variants in the oral cavity.
FundingThis work was supported by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq No. 303560/2012-6), Pro-Reitoria de Pesquisa e Pós-Graduação da Universidade Federal Fluminense (PROPPi-UFF), and Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ, No. E-26/111.255/2014). We would like to thank the Plataforma de Sequenciamento de DNA of the Universidade Federal Fluminense and André Victor Barbosa for technical assistance.
Conflicts of interestThe authors declare no conflicts of interest.