The current increment of invasive fungal infections and the availability of new broad-spectrum antifungal agents has increased the use of these agents by non-expert practitioners, without an impact on mortality. To improve efficacy while minimizing prescription errors and to reduce the high monetary cost to the health systems, the principles of pharmacokinetics (PK) and pharmacodynamics (PD) are necessary. A systematic review of the PD of antifungals agents was performed aiming at the practicing physician without expertise in this field. The initial section of this review focuses on the general concepts of antimicrobial PD. In vitro studies, fungal susceptibility and antifungal serum concentrations are related with different doses and dosing schedules, determining the PD indices and the magnitude required to obtain a specific outcome. Herein the PD of the most used antifungal drug classes in Latin America (polyenes, azoles, and echinocandins) is discussed.
Invasive fungal infections are currently an important cause of morbidity and mortality, especially in immunosuppressed patients and those admitted in intensive care units.1,2Candida spp. are the most frequent etiological agent of fungal infections in humans, ranking fourth among the etiologic agents of bloodstream infections in the United States, with a mortality similar to septic shock. In Latin America, a higher incidence of candidemia has been reported compared to countries of the northern hemisphere.3 Additionally, the diversity of climates and habitats in Latin America leads to a higher incidence of endemic mycoses, including histoplasmosis, paracoccidioidomycosis, and coccidioidomycosis.4
In the last years, the incidence of healthcare-associated fungal infections has been rising, mainly of invasive candidiasis and aspergillosis.5 There has also been an increased report of non-albicans Candida infections, which display reduced susceptibility to antifungal drugs.6 Finally, the number of patients with profound immunosuppression secondary to the treatment of hematologic malignancies and organ transplantation is growing, and it has been associated with the increase of invasive fungal infections due to several genera of molds that represent formidable diagnostic and therapeutic challenges.7 Despite the availability of effective antifungal drugs, mortality due to fungal infections remains high,8 a fact that has prompted the search for new products and a better understanding of the pharmacology of these agents to optimize therapy.
There are additional components in Latin America to the “host-fungi-drug” triad that may alter the pharmacodynamics (PD) with unknown impact on resistance. For economic and political reasons, generics are extensively used despite their unproven therapeutic equivalence and, in some cases, the treatment must be stopped due to shortage in supply. Moreover, the pharmacokinetics (PK)/PD knowledge has not been extensively introduced in the curriculum of medical schools and related clinical medical education activities are limited, mainly because PK and PD integrations may be seen as complex and not practical. In consequence, the advancements on the PD of antifungal have not been implemented as in other regions of the world.
MethodsA literature search was performed in the PubMed/MEDLINE database looking for clinical trials, journal articles or reviews available in full-text, written in Spanish or English languages, published from January 1962 to July 2015, including the keywords: pharmacodynamics (PD), pharmacokinetics (PK), antifungals, candidiasis, and aspergillosis. Papers about antifungal therapy co-authored by the expert in the field David R. Andes, were also searched. Out of 140 papers identified the authors selected the relevant papers about in vitro, in vivo, and clinical PD of antifungal agents, mainly emphasizing on the PK/PD concepts; to build a practical review for general physicians, especially in developing countries. The references of the selected papers were also used if the authors considered them relevant for the review.
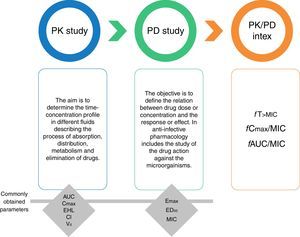
ReviewGeneral concepts of antimicrobial pharmacodynamicsThe study of the PK and PD properties of any antimicrobial is based on the exposure-response relationship of the drug and the infecting pathogen.9 This relationship can be modeled (Fig. 1) by integrating a PK parameter (e.g., maximal concentration or Cmax, and area under the curve or AUC) and a PD parameter related with the response expected against the infecting microorganism (i.e., minimal inhibitory concentration or MIC). The knowledge derived from these integrations has facilitated the design of optimal drug regimens and potentially reduce toxicity and the development of resistance.10
Pharmacokinetics (PK), pharmacodynamics (PD) and PK/PD integration. Pharmacokinetic parameters. AUC, area under the concentration-time curve; Cmax, maximal concentration or peak; EHL, elimination half-life; Cl, clearance; Vd, volume of distribution. Pharmacodynamic parameters. Emax, maximum effect, a measure of efficacy. ED50, effective dose to achieve 50% of the Emax, a measure of potency. MIC, minimal inhibitory concentration.
Classically, PK studies are about assessing absorption, distribution, metabolism, and elimination of drugs. The aim of PK is to determine the time-concentration drug profile in different fluids and the degree of penetration to different organs, because the drug effect is expected to correlate with the concentration at the site of the infection. Once a drug is inside of the human body, its movement between compartments and fluids depends on factors such as the fraction of unbound drug. For this reason, in addition to the total drug concentration, for PK studies it is necessary to determine the degree of protein binding as only unbound drug exerts pharmacological activity.11
PD studies, on the other hand, have to do with the relation between the drug concentration at site of action and the response or effect. In anti-infective pharmacology, PD studies integrate the susceptibility of the microorganisms (measured by the MICs) with the antimicrobial exposure (based on the PK data) and the observed effect or response. With this information it is possible to estimate PD indices (or PK/PD index) related to efficacy, as well as their level necessary for specific therapeutic goals. For antifungal drugs, three PD indices have been described: (i) the fraction of the dosing interval the free drug concentration is above the MIC (fT>MIC); (ii) the ratio of the area under the concentration-time curve and the MIC (fAUC/MIC); and (iii) the ratio of the maximum concentration or peak and the MIC (fCmax/MIC). During the last 15 years, these indices have been used to design optimal antifungal dosing regimens with known probability of success along a wide range of MIC and also to establish susceptibility breakpoints.12,13
To elucidate the PK/PD index for each antifungal, in vitro and in vivo studies are necessary. The process begins with in vitro susceptibility testing to determine the MIC under reproducible conditions,14 followed by a PK study to estimate the population parameters (clearance and volume of distribution). The final step is a dose-response experiment to relate exposure with antimicrobial effect using drug fractionation, that is, testing the same dose in multiple dosing schedules (e.g. q1h, q3h, q6h, q8h, q12h, and q24h). At the end, the three indices mentioned before (fT>MIC, fCmax/MIC and fAUC/MIC) are plotted against the effect to determine which index exhibits the best correlation with efficacy and to estimate the level required to achieve a particular endpoint (for instance, to obtain 50% of maximal efficacy or 90% survival). Thus, for drugs driven by the fCmax/MIC index (concentration-dependent), the dosing strategy is to use large doses with infrequent intervals, whereas for fT>MIC drugs (time-dependent) the optimal regimen would be smaller but more frequent doses or even extended or continuous infusion. In the case of drugs driven by fAUC/MIC, the key factor is the total amount of drug administered in 24h, independently of the dosing schedule used.
Another key factor to determine the PD indices is the persistent effect, i.e., the capacity of a drug to suppress the growth of the fungus after the compound is fully removed from the medium (the post-antifungal effect or PAFE), or after the concentration falls below the MIC (sub-MIC PAFE).15 Drugs driven by fCmax/MIC and fAUC/MIC exhibit prolonged PAFE whilst the fT>MIC driven ones usually display short or no PAFE. The duration of the PAFE depends on the type of action (fungicidal or fungistatic), the drug concentration and the pathogen being treated. For example, the echinocandins are fungicidal and exhibit long PAFE against Candida spp., while fungistatic and devoid of PAFE against Aspergillus fumigatus.16
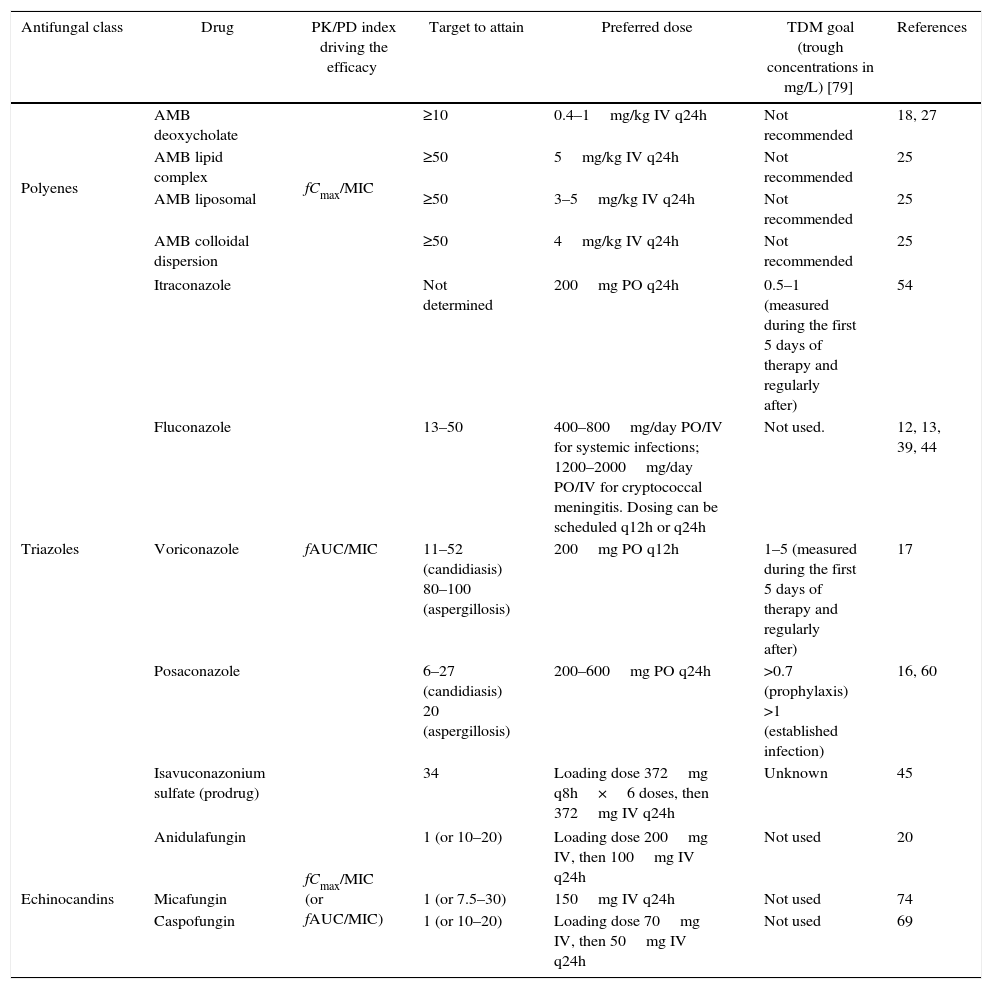
The systemic antifungal classes currently available are polyenes, triazoles, echinocandins, and flucytosine. For all of them, the PK/PD indices have been determined both in vitro and in vivo,17–21 and the results confirmed by clinical studies (Table 1), demonstrating that dose optimization to attain PD targets leads to higher clinical efficacy.22,23 Due to limited availability of flucytosine in developing countries, the present review will focus on the PD of polyenes, triazoles, and echinocandins.
Summary of the PK/PD indices and their required magnitude for efficacy with antifungal drugs and recommended goals for therapeutic drug monitoring (TDM).
| Antifungal class | Drug | PK/PD index driving the efficacy | Target to attain | Preferred dose | TDM goal (trough concentrations in mg/L) [79] | References |
|---|---|---|---|---|---|---|
| Polyenes | AMB deoxycholate | fCmax/MIC | ≥10 | 0.4–1mg/kg IV q24h | Not recommended | 18, 27 |
| AMB lipid complex | ≥50 | 5mg/kg IV q24h | Not recommended | 25 | ||
| AMB liposomal | ≥50 | 3–5mg/kg IV q24h | Not recommended | 25 | ||
| AMB colloidal dispersion | ≥50 | 4mg/kg IV q24h | Not recommended | 25 | ||
| Triazoles | Itraconazole | fAUC/MIC | Not determined | 200mg PO q24h | 0.5–1 (measured during the first 5 days of therapy and regularly after) | 54 |
| Fluconazole | 13–50 | 400–800mg/day PO/IV for systemic infections; 1200–2000mg/day PO/IV for cryptococcal meningitis. Dosing can be scheduled q12h or q24h | Not used. | 12, 13, 39, 44 | ||
| Voriconazole | 11–52 (candidiasis) 80–100 (aspergillosis) | 200mg PO q12h | 1–5 (measured during the first 5 days of therapy and regularly after) | 17 | ||
| Posaconazole | 6–27 (candidiasis) 20 (aspergillosis) | 200–600mg PO q24h | >0.7 (prophylaxis) >1 (established infection) | 16, 60 | ||
| Isavuconazonium sulfate (prodrug) | 34 | Loading dose 372mg q8h×6 doses, then 372mg IV q24h | Unknown | 45 | ||
| Echinocandins | Anidulafungin | fCmax/MIC (or fAUC/MIC) | 1 (or 10–20) | Loading dose 200mg IV, then 100mg IV q24h | Not used | 20 |
| Micafungin | 1 (or 7.5–30) | 150mg IV q24h | Not used | 74 | ||
| Caspofungin | 1 (or 10–20) | Loading dose 70mg IV, then 50mg IV q24h | Not used | 69 | ||
This antifungal class includes amphotericin B deoxycholate (AMBd) and the lipid formulations: lipid complex (AMBlc), colloidal dispersion (AMBcd), and liposomal (AMBl).
The mechanism of action requires its binding to the ergosterol in the fungal membrane, altering the cellular permeability and leading to cell death. All formulations of AMB display a concentration-dependent effect with prolonged PAFE, and the driving PK/PD index is the fCmax/MIC.15,16,19,24
Dose fractionation studies with the neutropenic mouse disseminated candidiasis (NMDC) model, confirmed that the fungicidal efficacy of AMBd correlated with large, infrequent doses; for instance, dosing AMBd every 72h (q72h) achieved maximal efficacy with doses 5–7 times lower than q6h or q12h schedules.19 For fungistatic and maximally fungicidal effects, the Cmax/MIC were 2–4 and 10, respectively. Similarly, but in the murine model of invasive pulmonary aspergillosis, q72h dosing schedules also led to a larger reduction in the fungal burden and longer survival of infected animals in comparison with q8h or q24h; in this model, the Cmax/MIC required for maximal efficacy was 2.4.25
In the NMDC model, AMBd was 4.3- to 5.9-fold more potent than AMBlc and AMBl in a mg/kg basis.26 Similarly, in the neutropenic-rabbit model of invasive pulmonary aspergillosis, near-maximal antifungal activity was evident with AMBd at 1mg/kg/day and AMBlc and AMBl at 5mg/kg/day.27 Thus, to achieve the needed Cmax/MIC with lipid formulations in clinical practice, the daily dose should be almost 5-times higher than the dose of AMBd. In opposition to the narrow therapeutic index of AMBd, the lipid formulations are significantly less toxic in relation to the dose of AMBd, specifically in terms of electrolytic imbalance, azotemia, renal tubular acidosis, anemia, and arrhythmias.28
Regarding the PK of polyenes, the concentration-time profile of amphotericin is nonlinear but there are important differences between AMBd and lipid formulations.29 AMBd is extensively distributed to tissues such as liver, kidneys, spleen and, in small quantities (<1%), to heart and brain, but clearance (Cl) from these sites is so slow (38±15mL/h/kg) as it takes more than one week to clear a single dose. Additionally, it has a volume of distribution (Vd) of 5±3L/kg and the protein binding is very high (>95%).30,31 AMB levels in cerebrospinal fluid and vitreous are null to minimal, therefore intrathecal or intravitreal administration is needed in selected cases.
The lipid vehicle in the other formulations of amphotericin B alters the distribution and clearance of the drug.30,32 For example, as AMBlc is a complex of AMB with lipid molecules, it displays the highest Vd (131±8L/kg) and fastest Cl (426±189mL/h/kg) of all formulations. In contrast, AMBl has the lowest Vd (0.11±0.08L/kg) and Cl (11±6mL/h/kg) among amphotericin formulations, while AMBcd has a similar Vd but faster clearance (117mL/h/kg) than AMBd. Additionally, as lipid forms accumulate more in the mononuclear phagocytic system and 10-times less in the kidneys, they are less frequently associated with nephrotoxicity than AMBd.33
Among the lipid formulations, AMBl is the smallest, unilamellar molecule (60–70nm vs. 1600–11,000nm for AMBlc), achieving the highest Cmax (83±35mg/L) after a single dose,34 but also displaying the highest protein binding. However, the small size of AMBl facilitates slightly higher concentrations in central nervous system compared to other lipid formulations. In fact, in the central nervous system model of candidiasis, only AMBl and AMBd sterilized the brain tissue, whereas the other lipid formulations did not,34 and only AMBd and AMBl reached detectable cerebrospinal fluid concentrations, with a transfer rate ranging from 0.02% to 0.92%.35
The clinical correlation of the potential PD differences among lipid formulations has been difficult to establish because there are limitations regarding the MIC determination and the differences in the models used to establish the PK/PD indices. Further, therapeutic monitoring is not generally advocated for AMB and there are no widely accepted drug exposure targets. However, the optimal target attainment has been defined for AMBd as well as AMBl. A PK/PD study in nine children with fungal infection treated with AMBl found that, while a Cmax/MIC=40±13 produced a partial response, full response needed it to be 67.9±17 (p=0.021).36 Considering that AMBd is approximately 5× more potent than AMBl, these results are consistent with the reported value of Cmax/MIC of 10 for maximal efficacy of AMBd.
Cornelly et al. conducted a multicenter randomized double-blind study in patients with probable or demonstrated mold invasive infections. Their study aimed to optimize the Cmax/MIC with AMBl comparing a 3mg/kg vs. a 10mg/kg dose based on a previous work that showed that the latter reaches Cmax>100mg/L. No significant differences were found in the global clinical response (50% vs. 46%) or survival after 12 weeks (71% vs. 58%), but there was a higher incidence of renal toxicity (16% vs. 30%).37 The absence of clinical benefit of the higher dose can be explained by the fact that at 3mg/kg the goal of the PK/PD index required for maximal efficacy has already been reached, and higher doses only lead to increased toxicity.30 Finally, a 999-patient simulation based in the neutropenic-rabbit model of invasive pulmonary aspergillosis showed that 3mg/kg/day of AMBl or 1mg/kg/day of AMBd was predicted to result in near-maximal antifungal activity and suppression of biomarkers.27 The optimal dose of AMBlc and AMBcd has not been as clearly established as the AMBd and AMBl.
TriazolesThese fungistatic drugs act blocking the synthesis of ergosterol mainly by inhibiting the cytochrome P450 dependent 14-α demethylase (CYP51A1), which converts lanosterol into ergosterol.38,39 These drugs are actually time-dependent, but they also exhibit prolonged PAFE, therefore the PD index driving efficacy is the fAUC/MIC.13,15,17,18,24,40
Fluconazole absorption from the gastrointestinal tract is almost complete and unaffected by gastric acidity or food, is poorly metabolized, and bioavailability is near 100%. Its elimination half-life ranges from 25 to 30h, it exhibits minimal protein binding (12%), and is excreted mainly by the kidneys. The Cmax obtained after a 100mg oral dose was 2mg/L.41 As fluconazole is a small molecule (MW=309) actively distributed into body fluids (Vd=46±8L), the levels attained in cerebrospinal fluid (or even in brain parenchyma) at steady state are ≥50% of the concentration observed in plasma. Due to this PK property, fluconazole is a well-accepted alternative therapy for cryptococcal (at high doses of 1200–2000mg/day) and Candida meningoencephalitis. Itraconazole, by contrast, is a large molecule (MW=705) highly bound to proteins, exhibiting a cerebrospinal fluid/plasma concentration ratio of ≤0.1242 that prevent its use in fungal CNS infections.
Regarding fluconazole, Louie et al. demonstrated in the murine model of systemic candidiasis that the effect of this drug on the fungal burden was the same when administering a single dose or dividing it in two or four injections.40 However, it should be noted that prolonged periods of exposure below the MIC might favor resistance of C. albicans against fluconazole.43,44 Multiple studies in vivo with fluconazole against several species of Candida along a wide MIC range have conclusively shown that the fAUC/MIC necessary to reach 50% of the maximal efficacy (ED50) is between 12.5 and 50.12,13,40 We determined in the NMDC model that two vastly different strains of C. albicans (MIC 0.25 and 4mg/L) required fAUC/MIC of 25–38 to attain the ED50.45
The relationship between fluconazole dose, the MIC of the infecting agent, and the outcome in patients with mucocutaneous candidiasis and candidemia has been addressed in several papers.12,22,23 Rex et al. published two decades ago that the clinical efficacy of fluconazole began to diminish with MIC above 8mg/L, but they did not run a PK/PD analysis.22 Later, Rodriguez-Tudela et al. studied 126 patients with candidemia and 110 with pharyngeal candidiasis finding that dose/MIC ratios of at least 100 (corresponding to fAUC/MIC=79) predicted cure for 93% of the subjects.22 In the Latin America Invasive Mycosis Network, the MIC90 for blood-isolated C. albicans, C. parapsilosis, and C. tropicalis were ≤1mg/L3; as those three strains represent 82% of cases, no more than 400mg/day of fluconazole should be necessary to treat most of patients.
With the other triazoles, similar fAUC/MIC ratios have been found against several Candida spp.17,18,46 For example, voriconazole and posaconazole require respectively fAUC/MIC of 11–52 and 6–27 to reach the ED50 in the NMDC model, while isavuconazole (protein binding>95%) required fAUC/MIC of 34 to achieve 90% survival, quite similar to other triazoles. Pharmacokinetic studies of voriconazole in humans have shown 58% protein binding and nonlinear saturable kinetics, with the AUC increasing in a higher proportion than the dose. At doses of 200mg PO q12h or 3mg/kg IV q12h, the fAUC is 20.47 Considering that the target for ED50 is fAUC/MIC around 20 (range 11–50), one could infer that clinical success would be attained with strains with MIC up to 1mg/L. This agrees with the susceptibility breakpoint defined by CLSI, highlighting the relevance of the PK/PD approach.48 All Candida species isolated in the Latin America Invasive Mycosis Network had a MIC≤0.5mg/L.
The pharmacodynamics of voriconazole has also been studied in the disseminated aspergillosis model (induced by intravenous inoculation).49 The fAUC/MIC required for 50% survival (ED50) was around 12, while for 100% survival (Emax) it went up to 80–100. Regarding posaconazole, Lepak et al. assessed its pharmacodynamics against Aspergillus spp. in the pulmonary invasive aspergillosis model (induced by nasal instillation),50 finding that the fAUC/MIC necessary to achieve the ED50 was 1.8, but at least 10 was required to reach the Emax. Howard et al. published similar results,51 and Conte et al. found in a study of pulmonary transplant patients52 that posaconazole concentration was 50 times higher in alveolar cells than in serum or epithelial lining fluid. These results suggest that the PK/PD profile of posaconazole is favorable for the treatment of pulmonary aspergillosis.
There are also pharmacodynamic data with voriconazole in patients with invasive aspergillosis, a frequent indication of this drug. The recommended trough levels of voriconazole for therapeutic drug monitoring (TDM) correlating with lower toxicity and better survival outcomes range from 1 to 2mg/L53; however, a large inter-individual variability in the kinetics of the drug was observed. In consequence, 25% of patients achieved trough levels<1mg/L and therapy failed, whilst 31% had levels>5.5mg/L and caused toxicity. Based on these data, a randomized clinical study was carried out to assess the impact of TDM of voriconazole in 110 patients with invasive fungal infections. The trial showed that TDM was associated with higher efficacy (81% vs. 57%, p=0.04) and lower drug discontinuation due to toxicity (4% vs. 17%, p=0.02).54 These results support the recent recommendations by the British Society for Medical Mycology indicating TDM for all patients receiving voriconazole. The goal is to reach trough levels (Cmin) between 1 and 4–6mg/L or, ideally, a Cmin/MIC ratio of 2–5.55
Posaconazole is characterized by a long half-life (approximately 30h), saturable absorption at 800mg/day, high protein binding (98%), and a long time to reach steady state concentrations (10 days).56 It is usually administered as a suspension twice a day, so the concentration-time curve is almost flat with very similar peaks and troughs. Among 67 patients with refractory invasive aspergillosis that received posaconazole and underwent TDM, it was found that those with peak (Cmax) and average concentrations (Cavg) of 0.14 and 0.13mg/L, respectively, only had 24% response. In contrast, in patients with Cmax and Cavg of 1.48 and 1.25mg/L, 75% had a favorable response.57 These results support the recommendation of trough levels>1mg/L for patients with established infections and 0.7mg/L for prophylaxis.55 The PK of posaconazole also exhibits high between-subject variability in patients with hematologic malignancies,58 another reason for TDM. To the best of our knowledge, there are no clinical studies correlating the fAUC/MIC of posaconazole with clinical outcome, but considering that 800mg per day yields fAUC around 0.30–0.44,52,59 a fAUC/MIC near 3 can be attained with MICs up to 0.125mg/L, the EUCAST susceptibility breakpoint for A. fumigatus.60 To improve bioavailability and reduce between-subject variability in patients, a delayed-release (DR) tablet formulation was developed. At a dose of 300mg once a day (three 100mg DR tablets), 90% of patients achieved posaconazole serum concentrations greater than 700ng/mL, compared with 58% patients receiving the oral suspension. Acid suppression did not affect posaconazole levels in the DR tablet cohort.61
EchinocandinsThese are large lipoproteins with negligible oral bioavailability, extensive protein binding (>95%), lack of renal clearance, and no penetration to cerebrospinal fluid. Their unique mechanism of action (inhibition of 1,3-β-glucan synthase), high in vivo efficacy, and safety profile, has increased its use as treatment for invasive candidiasis and aspergillosis. The three approved agents are caspofungin, anidulafungin, and micafungin.
Being the newer group of antifungals, echinocandins have been studied thoroughly from a PK/PD perspective.62 They are fungicidal against Candida spp. and fungistatic against Aspergillus spp. The PD index that best drive their action is the fCmax/MIC, displaying prolonged PAFE.21,63–65 However, in the NMDC model, the fAUC/MIC ratio is also a strong predictor of efficacy, presumably due to the prolonged tissue distribution of these compounds including the kidneys, where the antifungal effect is measured in this model. Dose fractionation studies have shown that the dose required to reduce the fungal burden by 1 log10 is 4-fold lower when the drugs are administered once daily.21,62 Of note, a paradoxical effect consisting in a reduced fungicidal efficacy against Candida spp. has been observed in vitro at concentrations above the MIC.66 Such effect results of compensatory responses to 1,3-β-glucan synthesis and disappears when human serum is added, indicating that it is not likely to impact the treatment of patients with candidemia.67
The magnitude of PD indices bound to maximal efficacy in animal models of invasive candidiasis is a fCmax/MIC of 1 or a fAUC/MIC between 10 and 20 for anidulafungin,21 micafungin,62 and caspofungin. Considering that the usual dose of anidulafungin (200mg loading dose followed by 100mg q24h) is associated with fCmax=1.15mg/L and fAUC=1.12mghL−1,68 the PD target will be attained against strains with MIC≤0.125mg/L. Recent epidemiological studies indicate that the MIC90 of anidulafungin is <0.125mg/L for many Candida species excepting C. parapsilosis, C. guilliermondii, C. lusitaniae and C. famata.69 An in vivo PD study found that the fAUC/MIC to be fungistatic against C. parapsilosis and C. glabrata was 7, in contrast to 20 against C. albicans.70 These data support the CLSI susceptibility breakpoint of 0.25mg/L for C. albicans, C. tropicalis, and C. krusei, and 2mg/L for C. parapsilosis.71
The majority of clinical trials with echinocandins have focused on the evaluation of clinical efficacy72,73 and its relation to the MIC.74 Regarding micafungin, a PK/PD analysis of data from 493 patients found that a fAUC/MIC between 7.5 and 30 was associated with 98% microbiological success, compared to 85% when the index was <7.5.75 Finally, in patients with C. parapsilosis infection, a fAUC/MIC>0.71 was related to 100% microbiological success compared with 82% in patients with values<0.71. This is a further demonstration that C. parapsilosis requires a 10-fold lesser fAUC/MIC, in line with its higher susceptibility breakpoint.
Finally, in pediatric patients, dosing adjustment is necessary for most antifungal drugs to obtain the same exposure achieved in adults. Also, differences are known in drug metabolism and clearance, and the magnitude of the PK/PD indices requires more studies in that specific population. Fungal infections in children compared to adults may affect organs and systems differently (e.g. CNS infection during candidemia is frequent in children). A recent review on pediatric pharmacology of antifungal agents is available.76
Conclusion and future perspectivesFungal diseases are a growing public health problem. Although epidemiological data are scant, a prospective survey shows an incidence of 0.98 per 1000 hospital admissions in Latin America, approximately four-times the incidence reported in Europe and North America.77 Medical comorbidities and political, economic, and climatic factors are contributing to increase and to perpetuate the problem. In addition, antifungal pharmacology knowledge has been progressively increasing, mainly, by the determination of drug exposure-response relationships. The integration of PK/PD has facilitated the design of optimal drug regimens that help maximize efficacy while simultaneously reducing toxicity, ameliorating the development of resistance and cutting costs, as corroborated by clinical trials.78 This knowledge can be applied to each patient in any clinical setting (Table 1), although TDM is necessary to assure that the magnitude of the PK/PD indices required for maximal efficacy are effectively achieved. This process would introduce an additional expenditure related to the quantification of drugs in specialized laboratories, which could be offset by the much greater economic and social benefits of optimized therapy.79
Author's contributionJMG made the search of literature, reviewed the papers included, and wrote the first draft of the manuscript. CAR, MA and AZ reviewed the literature, made criticism to the article and approved the final version. OV reviewed and edited the final text.
Conflicts of interestGonzalez, Agudelo and Vesga have no conflicts of interest to declare. Rodriguez has received honorary for unrelated lectures from Roche and Amgen. Zuluaga has received honorary for unrelated lectures from Allergan, Amgen, Lilly, Mundipharma, Novo Nordisk, Pfizer, Roche and Sanofi. None of these companies or any other pharmaceutical company was involved in the design, execution, or publication of this study.
Funding for this project came from the Universidad de Antioquia (UdeA) and Sistema General Regalías of Colombia (BPIN: 2013000100183). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.