Clostridioides difficile infection (CDI) is the most common cause of healthcare-associated infections in Western countries. Risk factors, mortality, and healthcare utilization for CDI in Latin America are poorly understood. This study assessed risk factors and burden associated with nosocomial CDI in four Latin American countries.
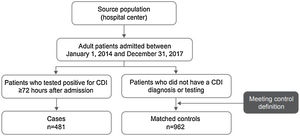
MethodsThis retrospective, case-control study used databases and medical records from 8 hospitals in Argentina, Brazil, Chile, and Mexico to identify nosocomial CDI cases from 2014 − 2017. Cases were patients aged ≥18 years with diarrhea and a positive CDI test ≥72 h after hospital admission. Two controls (without diarrhea; length of hospital stay [LOS] ≥3 days; admitted ±14 days from case patient; shared same ward) were matched to each case. CDI-associated risk factors were assessed by univariate and multivariable analyses. CDI burden (LOS, in-hospital mortality) was compared between cases and controls.
ResultsThe study included 481 cases and 962 controls. Mean age and sex were similar between cases and controls, but mean Charlson comorbidity index (4.3 vs 3.6; p < 0.001) and recent hospital admission (35.3% vs 18.8%; p < 0.001) were higher among cases. By multivariable analyses, CDI risk was associated with prior hospital admission within 3 months (odds ratio [OR], 2.08; 95% CI: 1.45, 2.97), recent antibiotic use (ie, carbapenem; OR, 2.85; 95% CI: 1.75, 4.64), acid suppressive therapy use (OR, 1.71; 95% CI: 1.14, 2.58), and medical conditions (ie, renal disease; OR, 1.48; 95% CI: 1.19, 1.85). In-hospital mortality rate (18.7% vs 6.9%; p < 0.001) and mean overall LOS (33.5 vs 18.8 days; p < 0.001) were higher and longer, respectively, in cases versus controls.
ConclusionAntibiotic exposure, preexisting medical conditions, and recent hospital admission were major risk factors for CDI in Argentina, Brazil, Chile, and Mexico. CDI was associated with increased in-hospital risk of death and longer LOS. These findings are consistent with published literature in Western countries.
Clostridioides difficile is an anaerobic, Gram-positive bacterium that undergoes fecal–oral transmission leading to colonization of the large intestine and release of protein exotoxins.1C. difficile infection (CDI) can manifest as a broad spectrum of symptoms, ranging from mild diarrhea to serious and life-threatening conditions, such as pseudomembranous colitis and toxic megacolon.1,2
Antibiotic exposure, which alters the natural flora of the intestines and in turn allows the bacterium to proliferate, is the most important risk factor for CDI.1,3 Other factors associated with CDI include advanced age, prior healthcare exposure, and increased number of comorbid conditions.3
The epidemiology of CDI is temporally and geographically variable; however, incidence rates have generally been increasing over the past 20 years.1,4 In high-income countries, CDI has been most common in healthcare settings and is the most frequent cause of hospital-acquired infectious diarrhea and healthcare-associated infections.5–7 Notably, a growing proportion of CDI cases are now community associated.1,8 In 2017, the incidence of all CDIs, healthcare-associated CDIs, and community-associated CDIs in the United States was 130.3, 67.0, and 63.3 per 100,000, respectively; in EU countries participating in healthcare-associated infections surveillance, the mean hospital incidence density was 3.2 and 2.4 per 10,000 patient-days for total CDI and hospital-associated CDI, respectively, in 2016.8,9 Additionally, a recent meta-analysis estimated 2.24 healthcare-associated CDI cases per 1000 admissions based on reports published between 2005 and 2015 from 41 countries, most of which were from Europe and North America.10
CDI can result in substantial morbidity and mortality1,11 and is associated with prolonged length of stay (LOS) in the hospital, as well as other healthcare utilizations, including direct costs for treating the patient and indirect costs to prevent spread of the infection.11 Additionally, although antibiotic treatment of acute CDI infections is indicated, including use of metronidazole and vancomycin, continued antibiotic treatment during and after initial CDI infection can result in poor outcomes, including recurrence.1,12,13 Concurrently with the observed increase in CDI incidence rates, CDI associated mortality has risen in the past two decades.4,14 Based predominantly on Western data sets, mortality from CDI is estimated at 6.0% and is highest in older individuals.14
As available investigations of the epidemiology of CDI have focused on resource-rich settings, comparatively less is known about the burden in other regions.15 In a recent review of the epidemiology of CDI in low- and middle-income countries, the prevalence of CDI varied considerably in South America, which is likely attributed to differences in diagnostic approaches, study populations, and study design. In a systematic review and meta-analysis of CDI epidemiology in developing countries among patients with diarrhea, the prevalence of a first episode of community- and hospital-onset CDI was 19% (95% CI: 13, 27) in Latin America compared with a prevalence of 15% (95% CI: 13, 17) among all regions included in the analysis (ie, Africa/Middle East, developing countries in Asia, Latin America, and China).16
Unfortunately, little is known about CDI occurring in Latin America because of a paucity of published data.17 Available studies from Latin America have focused predominantly on CDI incidence rates,18–28 and limited data are available on risk factors associated with CDI or its consequences, such as mortality and healthcare utilization. Therefore, this hospital-based, nested case-control study assessed the risk factors and burden, including in-hospital mortality and LOS, associated with nosocomial CDI in four countries in Latin America.
Material and methodsDesignThe study was a retrospective, multicenter, hospital-based, case-control assessment conducted at eight hospital centers in Argentina, Brazil, Chile, and Mexico. Hospital databases and medical records (either electronic- or paper-based per center standards) were used to identify all eligible nosocomial CDI cases and to obtain information regarding demographics, medical condition, drug history, prior healthcare exposure, and outcomes. Besides the microbiological approach to detect CDI, additional information on hospital-specific diagnostic practices (eg, clinical laboratory support to isolate the organism) was not collected. According to the case-control design, identified cases of nosocomial CDI were matched in a 1:2 ratio to controls selected from the same hospital among patients who had not developed CDI during hospitalization.
Case and control definitionTo be included as a CDI case patients had to be ≥18 years, have diarrhea, and have ≥1 positive CDI test from the following assays: glutamate dehydrogenase (GDH) + enzyme-linked immunosorbent assay (ELISA), ELISA alone, and/or polymerase chain reaction (PCR) occurring ≥72 h after hospital admission from January 1, 2014, to December 31, 2017. Patients with incomplete medical records (eg, lack of drug history) were excluded.
Two controls from the same hospital were randomly selected and matched to each case. Controls were required to be hospitalized patients not experiencing diarrhea who were at similar hospital exposure to the case at the time of diagnosis. The similar hospital exposure was defined as admitted to the same hospital center ±14 days from the counterpart CDI case admission and sharing the same ward as the case patient. To limit the patients not at risk or at low risk of nosocomial CDI, controls with a hospital stay <3 days were excluded. Patients could be selected as controls only once and could not be included as a control if they subsequently developed CDI after confirmatory diagnosis of nosocomial CDI, as described previously.
OutcomesThe risk factor analysis assessed the association of demographics, baseline clinical conditions, drug history, and prior healthcare exposure on CDI diagnosis between cases and controls. The comorbidities were measured by the Charlson comorbidity index (CCI), with a higher score indicative of greater comorbid condition.29 Recent drug history was recorded, including use of antibiotics, acid suppressants, immunosuppressants, enteral feeding, and lactulose within 60 days before index hospitalization. Type of systemic antibiotic by drug class was collected. Total duration of antibiotic therapy received within 60 days of index hospitalization was calculated as the total cumulative duration of antibiotic therapy before CDI diagnosis. Prior healthcare exposure included hospitalization within 90 days before index hospitalization.
Total LOS and in-hospital mortality were compared between cases and controls. The total LOS was calculated from the period between hospital admission and hospital discharge or in-hospital death, whichever occurred first. The in-hospital mortality was death during hospitalization as noted in the case report form. The management of CDI cases was also evaluated, including severity of CDI, antibiotic treatment, treatment cure or failure rate, and recurrence. The ATLAS score,30 which combines five clinical and laboratory variables into an 11-category scoring system, was used to assess CDI severity. Cure was defined as the complete disappearance of clinical, radiologic, and microbiological signs (i.e., repeated negative cultures) of CDI at the time of hospital discharge. Treatment failure was defined as persistence or incomplete resolution of symptoms or positive toxin assay after 10 days of treatment, whereas recurrence was defined as a second episode of CDI occurring within two to eight weeks of the index case within hospital stay.
Statistical analysesDescriptive statistics were used to summarize the data. Continuous and categorical variables for the univariate analysis were compared between groups using a generalized linear model and Cochran-Mantel-Haenszel chi-square test, respectively, controlled by hospital center.
Multivariate analyses were used to identify independent risk factors of CDI by applying conditional logistic regression models. When performing multivariate analyses, only those explanatory variables resulting in p-values less than the cut-off point of 0.20 in the univariate analysis were incorporated in the model with a stepwise method. To allow the assessment of individual agents, individual class of antibiotics was incorporated in the multivariate model. Models were assessed for goodness-of-fit, multicollinearity, and influential observations. Odds ratios (ORs) were calculated with their corresponding 95% confidence intervals (CIs), with the latter used to determine the association between the various independent variables and CDI diagnosis.
For analysis of burden associated with CDI, differences in total LOS between groups were used to calculate excess LOS attributable to CDI. Total LOS was compared using a generalized linear model, controlled by hospital center. In-hospital mortality was set as the outcome variable, and its association with underlying diseases, among other explanatory variables, was assessed between groups. A multivariate model was built using conditional logistic regression to identify in-hospital mortality predictors. Variables were selected using a stepwise method. All tests were two-sided, and p-values <0.05 were considered statistically significant. All analyses were performed using SAS version 9.4 (SAS Institute Inc, Cary, NC).
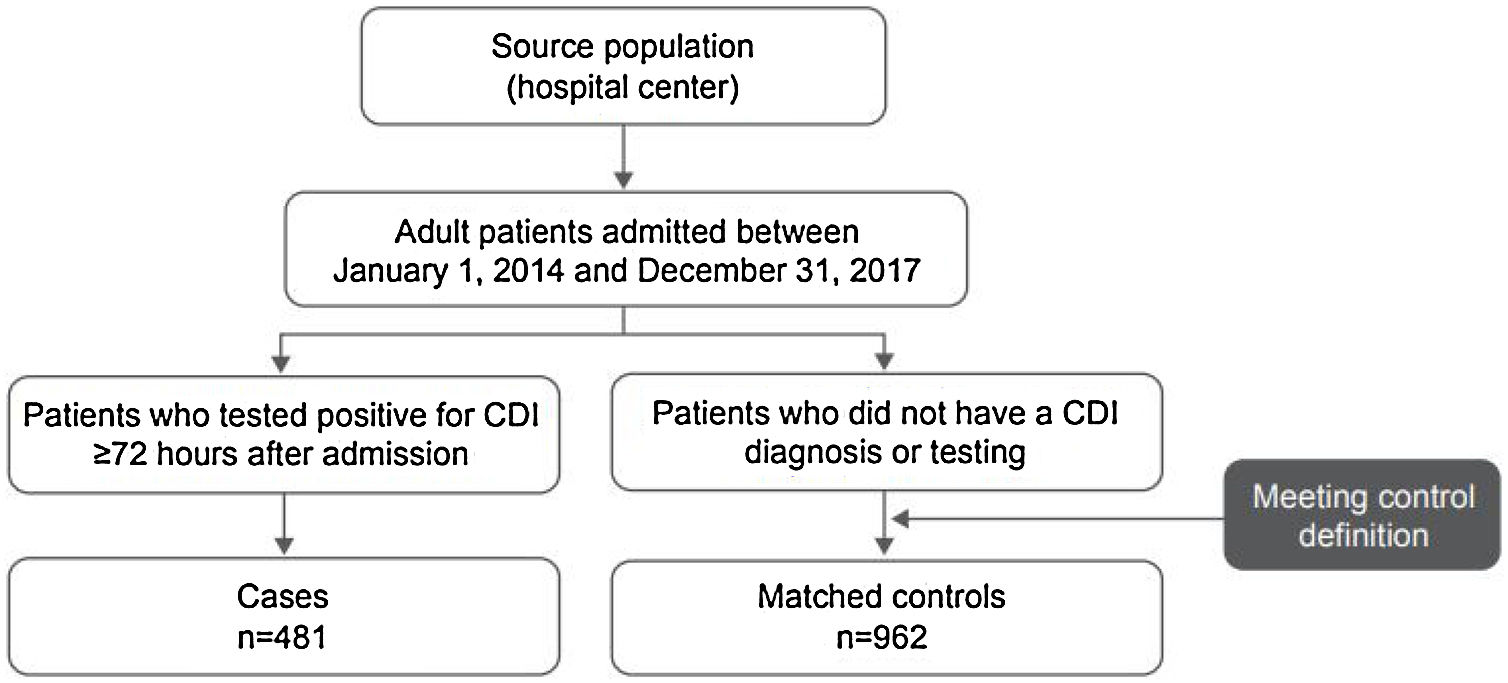
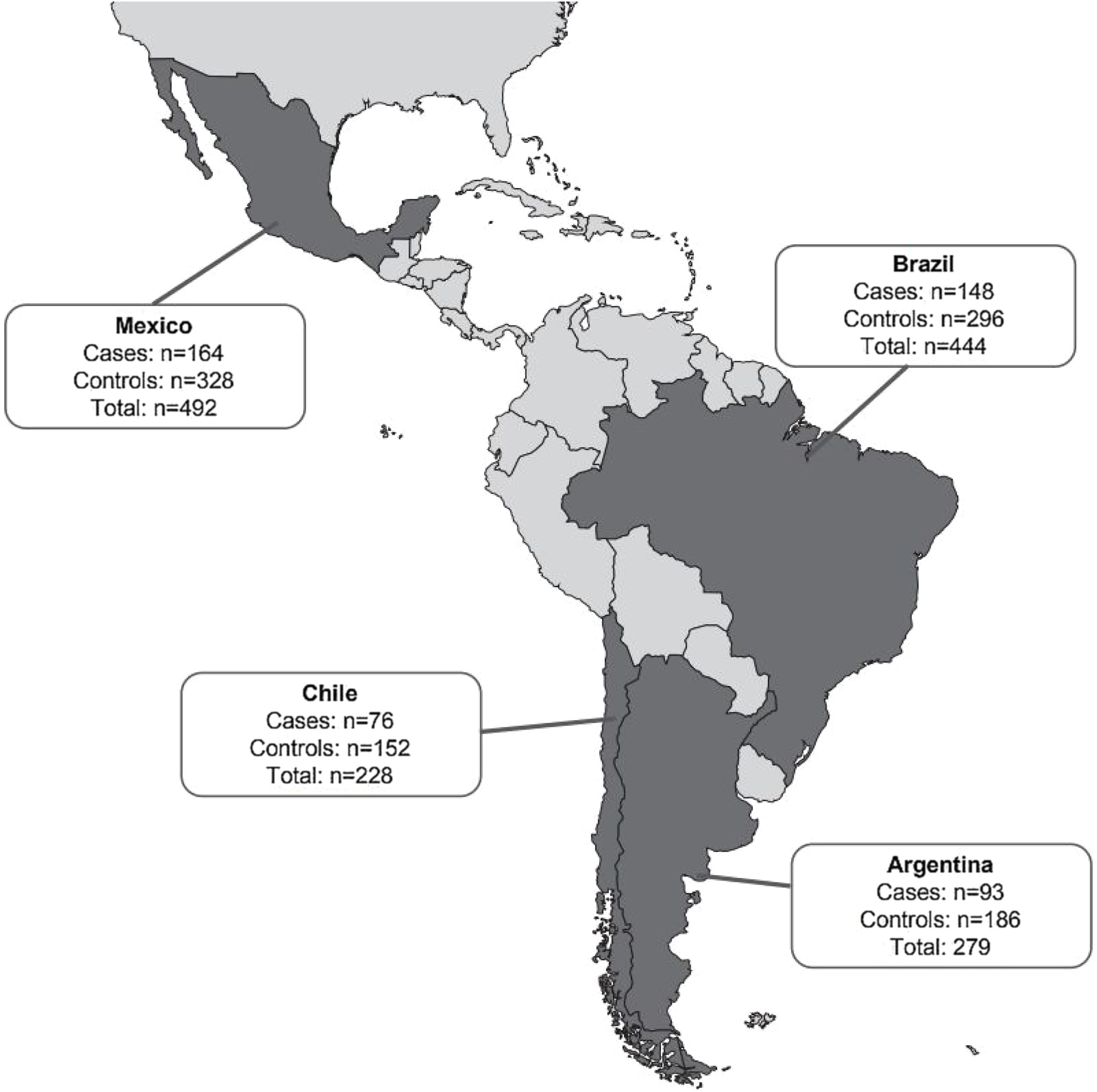
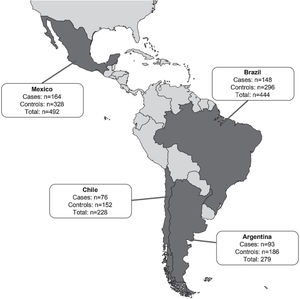
ResultsPatientsA total of 1443 patients (≥18 years of age) who met the case and control definitions were included in the study, comprising 481 CDI cases and 962 matched controls (Fig. 1). Nearly two-thirds of patients included in this analysis were identified in Mexico (34.1%; 492/1443) and Brazil (30.8%; 444/1443) during the study period (Fig. 2). Among the cases, CDI was most frequently confirmed by PCR testing (45.3%; 218/481) followed by ELISA alone testing (32.4%; 156/481) and GDH + ELISA testing (15.8%; 76/481) in the stool samples.
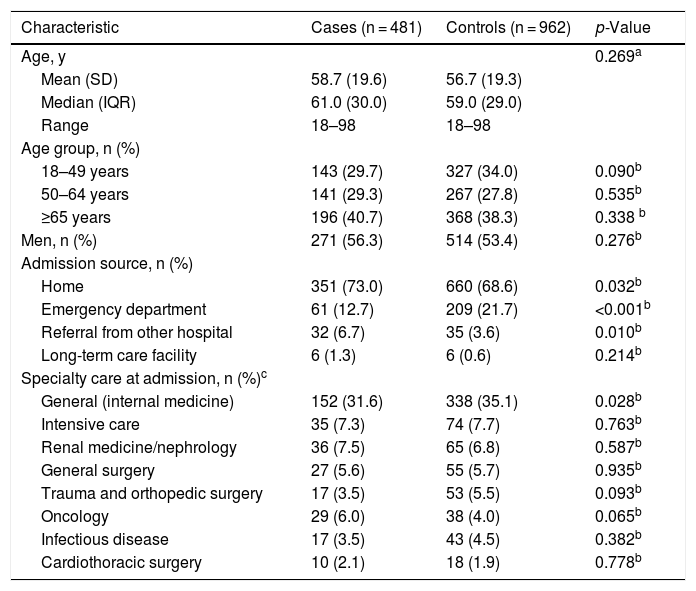
The majority of patients (67.4%; 972/1443) were 50 years and older, with a similar mean age between cases and controls (58.7 vs 56.7 years, p = 0.269) (Table 1). There were no significant differences between study groups regarding patient sex.
Patient Demographic Characteristics.
| Characteristic | Cases (n = 481) | Controls (n = 962) | p-Value |
|---|---|---|---|
| Age, y | 0.269a | ||
| Mean (SD) | 58.7 (19.6) | 56.7 (19.3) | |
| Median (IQR) | 61.0 (30.0) | 59.0 (29.0) | |
| Range | 18–98 | 18–98 | |
| Age group, n (%) | |||
| 18–49 years | 143 (29.7) | 327 (34.0) | 0.090b |
| 50–64 years | 141 (29.3) | 267 (27.8) | 0.535b |
| ≥65 years | 196 (40.7) | 368 (38.3) | 0.338 b |
| Men, n (%) | 271 (56.3) | 514 (53.4) | 0.276b |
| Admission source, n (%) | |||
| Home | 351 (73.0) | 660 (68.6) | 0.032b |
| Emergency department | 61 (12.7) | 209 (21.7) | <0.001b |
| Referral from other hospital | 32 (6.7) | 35 (3.6) | 0.010b |
| Long-term care facility | 6 (1.3) | 6 (0.6) | 0.214b |
| Specialty care at admission, n (%)c | |||
| General (internal medicine) | 152 (31.6) | 338 (35.1) | 0.028b |
| Intensive care | 35 (7.3) | 74 (7.7) | 0.763b |
| Renal medicine/nephrology | 36 (7.5) | 65 (6.8) | 0.587b |
| General surgery | 27 (5.6) | 55 (5.7) | 0.935b |
| Trauma and orthopedic surgery | 17 (3.5) | 53 (5.5) | 0.093b |
| Oncology | 29 (6.0) | 38 (4.0) | 0.065b |
| Infectious disease | 17 (3.5) | 43 (4.5) | 0.382b |
| Cardiothoracic surgery | 10 (2.1) | 18 (1.9) | 0.778b |
IQR = interquartile range; SD = standard deviation.
The most frequent source of admission was the patient’s home (70.1%; 1011/1443), with a significantly higher predominance in the case (73.0%; 351/481) versus control (68.6%; 660/962) group (p = 0.032) (Table 1). Additionally, hospital referral (i.e., transfer of a patient between hospitals) as the source of patient admission occurred more frequently among cases (6.7%; 32/481) versus control (3.6%; 35/962) group (p = 0.010), whereas patient admission from an emergency department was more common in the control group (21.7%; 209/962) than among cases (12.7%; 61/481) [p<0.001].
After admission, the medical specialty where the patient received treatment was relatively balanced between cases and controls (Table 1). A slightly lower percentage of cases (31.6%; 152/481) than control group (35.1%; 338/962) received internal medicine care on admission (p = 0.028). No other statistically significant differences between groups regarding speciality treatment received upon admission were observed.
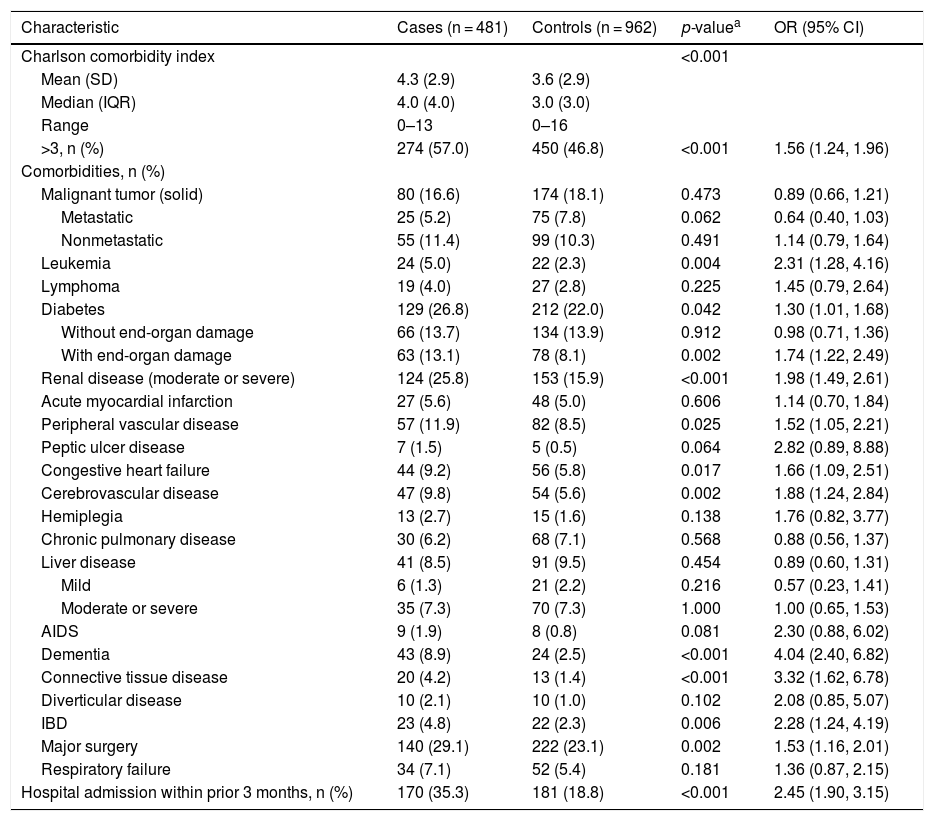
Clinical characteristicsPatients in the case group presented with a poorer baseline comorbid condition than their matched controls (Table 2). Mean CCI was significantly higher among cases (4.3) versus control group (3.6), respectively (p < 0.001), as was the percentage of patients with a CCI >3 (57.0%; 274/481 vs 46.8%; 450/962, respectively; p < 0.001). Major surgery, moderate or severe renal disease, diabetes with end-organ damage, peripheral vascular disease, cerebrovascular disease, congestive heart failure, dementia, leukemia, inflammatory bowel disease, and connective tissue disease were all significantly more frequent in the case versus control group. The percentage of patients in the case group with a recent hospital admission (<3 months before the index hospitalization) was significantly higher than in the control group (35.3%; 170/481 vs 18.8%; 181/962), respectively (p < 0.001). The ORs generated from univariate analyses showed consistent results.
Patient Baseline Clinical Characteristics.
| Characteristic | Cases (n = 481) | Controls (n = 962) | p-valuea | OR (95% CI) |
|---|---|---|---|---|
| Charlson comorbidity index | <0.001 | |||
| Mean (SD) | 4.3 (2.9) | 3.6 (2.9) | ||
| Median (IQR) | 4.0 (4.0) | 3.0 (3.0) | ||
| Range | 0–13 | 0–16 | ||
| >3, n (%) | 274 (57.0) | 450 (46.8) | <0.001 | 1.56 (1.24, 1.96) |
| Comorbidities, n (%) | ||||
| Malignant tumor (solid) | 80 (16.6) | 174 (18.1) | 0.473 | 0.89 (0.66, 1.21) |
| Metastatic | 25 (5.2) | 75 (7.8) | 0.062 | 0.64 (0.40, 1.03) |
| Nonmetastatic | 55 (11.4) | 99 (10.3) | 0.491 | 1.14 (0.79, 1.64) |
| Leukemia | 24 (5.0) | 22 (2.3) | 0.004 | 2.31 (1.28, 4.16) |
| Lymphoma | 19 (4.0) | 27 (2.8) | 0.225 | 1.45 (0.79, 2.64) |
| Diabetes | 129 (26.8) | 212 (22.0) | 0.042 | 1.30 (1.01, 1.68) |
| Without end-organ damage | 66 (13.7) | 134 (13.9) | 0.912 | 0.98 (0.71, 1.36) |
| With end-organ damage | 63 (13.1) | 78 (8.1) | 0.002 | 1.74 (1.22, 2.49) |
| Renal disease (moderate or severe) | 124 (25.8) | 153 (15.9) | <0.001 | 1.98 (1.49, 2.61) |
| Acute myocardial infarction | 27 (5.6) | 48 (5.0) | 0.606 | 1.14 (0.70, 1.84) |
| Peripheral vascular disease | 57 (11.9) | 82 (8.5) | 0.025 | 1.52 (1.05, 2.21) |
| Peptic ulcer disease | 7 (1.5) | 5 (0.5) | 0.064 | 2.82 (0.89, 8.88) |
| Congestive heart failure | 44 (9.2) | 56 (5.8) | 0.017 | 1.66 (1.09, 2.51) |
| Cerebrovascular disease | 47 (9.8) | 54 (5.6) | 0.002 | 1.88 (1.24, 2.84) |
| Hemiplegia | 13 (2.7) | 15 (1.6) | 0.138 | 1.76 (0.82, 3.77) |
| Chronic pulmonary disease | 30 (6.2) | 68 (7.1) | 0.568 | 0.88 (0.56, 1.37) |
| Liver disease | 41 (8.5) | 91 (9.5) | 0.454 | 0.89 (0.60, 1.31) |
| Mild | 6 (1.3) | 21 (2.2) | 0.216 | 0.57 (0.23, 1.41) |
| Moderate or severe | 35 (7.3) | 70 (7.3) | 1.000 | 1.00 (0.65, 1.53) |
| AIDS | 9 (1.9) | 8 (0.8) | 0.081 | 2.30 (0.88, 6.02) |
| Dementia | 43 (8.9) | 24 (2.5) | <0.001 | 4.04 (2.40, 6.82) |
| Connective tissue disease | 20 (4.2) | 13 (1.4) | <0.001 | 3.32 (1.62, 6.78) |
| Diverticular disease | 10 (2.1) | 10 (1.0) | 0.102 | 2.08 (0.85, 5.07) |
| IBD | 23 (4.8) | 22 (2.3) | 0.006 | 2.28 (1.24, 4.19) |
| Major surgery | 140 (29.1) | 222 (23.1) | 0.002 | 1.53 (1.16, 2.01) |
| Respiratory failure | 34 (7.1) | 52 (5.4) | 0.181 | 1.36 (0.87, 2.15) |
| Hospital admission within prior 3 months, n (%) | 170 (35.3) | 181 (18.8) | <0.001 | 2.45 (1.90, 3.15) |
IBD = inflammatory bowel disease; IQR = interquartile range; OR = odds ratio; SD = standard deviation.
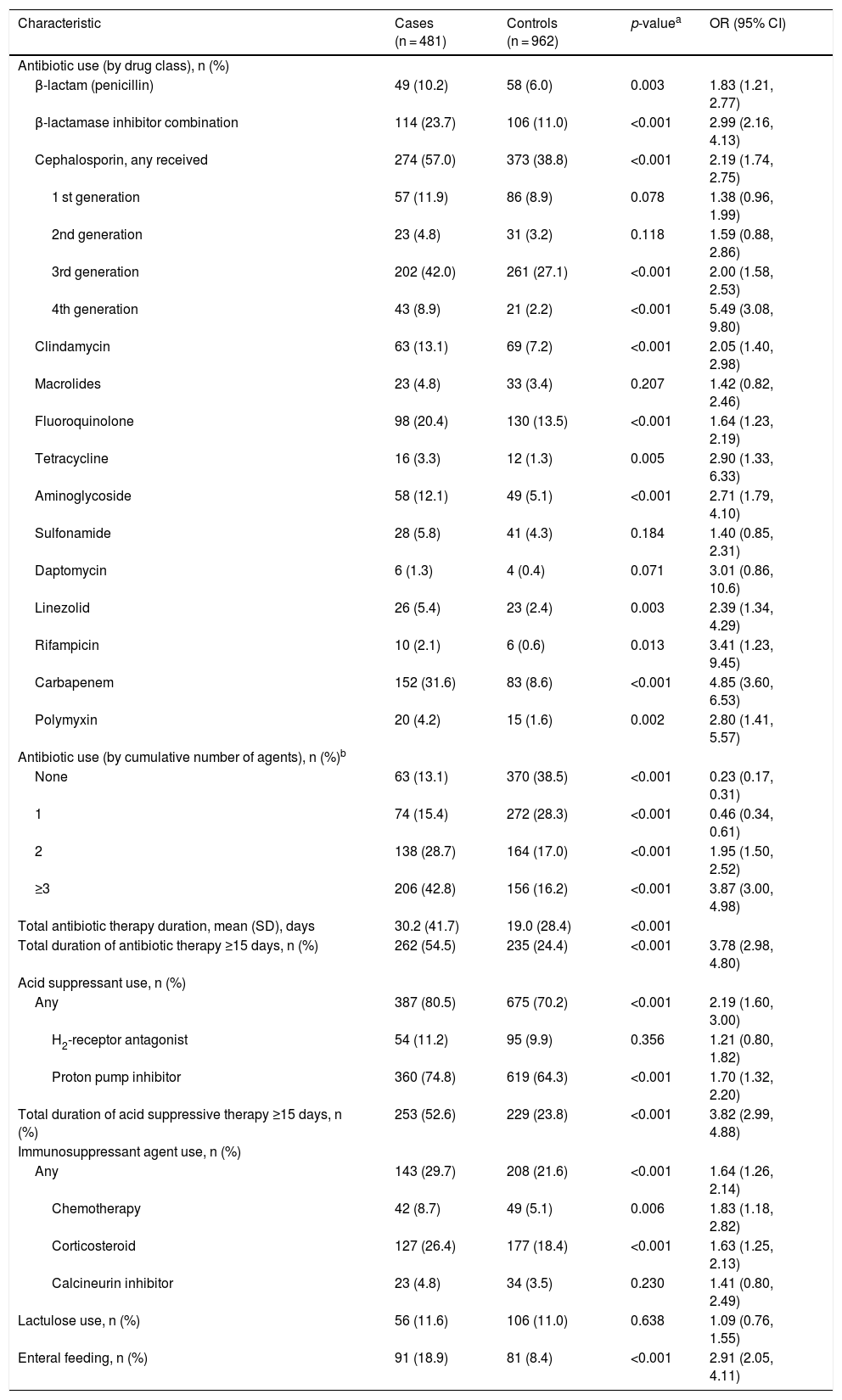
Higher percentages of patients in the case versus control group received systemic antibiotic treatment (excluding vancomycin and metronidazole) across all antibiotic classes within the 60 days before index hospitalization (Table 3). A higher percentage of cases (28.7%; 138/481 and 42.8%; 206/481) had received 2 or ≥3 systemic antibiotics, respectively, compared to the control group (17.0%; 164/962 and 16.2%; 156/962) respectively (p < 0.001 for both). Conversely, the percentage of patients in the case group (13.1%; 63/481 and 15.4%; 74/481) who had received no antibiotic or a single antibiotic in this period, respectively, was lower compared to the control group (38.5%; 370/962 and 28.3%; 272/962), respectively (p < 0.001 for both). No single antibiotic agent was received significantly more frequently by the control group compared with the case group.
Patient Baseline Drug History.
| Characteristic | Cases (n = 481) | Controls (n = 962) | p-valuea | OR (95% CI) |
|---|---|---|---|---|
| Antibiotic use (by drug class), n (%) | ||||
| β-lactam (penicillin) | 49 (10.2) | 58 (6.0) | 0.003 | 1.83 (1.21, 2.77) |
| β-lactamase inhibitor combination | 114 (23.7) | 106 (11.0) | <0.001 | 2.99 (2.16, 4.13) |
| Cephalosporin, any received | 274 (57.0) | 373 (38.8) | <0.001 | 2.19 (1.74, 2.75) |
| 1 st generation | 57 (11.9) | 86 (8.9) | 0.078 | 1.38 (0.96, 1.99) |
| 2nd generation | 23 (4.8) | 31 (3.2) | 0.118 | 1.59 (0.88, 2.86) |
| 3rd generation | 202 (42.0) | 261 (27.1) | <0.001 | 2.00 (1.58, 2.53) |
| 4th generation | 43 (8.9) | 21 (2.2) | <0.001 | 5.49 (3.08, 9.80) |
| Clindamycin | 63 (13.1) | 69 (7.2) | <0.001 | 2.05 (1.40, 2.98) |
| Macrolides | 23 (4.8) | 33 (3.4) | 0.207 | 1.42 (0.82, 2.46) |
| Fluoroquinolone | 98 (20.4) | 130 (13.5) | <0.001 | 1.64 (1.23, 2.19) |
| Tetracycline | 16 (3.3) | 12 (1.3) | 0.005 | 2.90 (1.33, 6.33) |
| Aminoglycoside | 58 (12.1) | 49 (5.1) | <0.001 | 2.71 (1.79, 4.10) |
| Sulfonamide | 28 (5.8) | 41 (4.3) | 0.184 | 1.40 (0.85, 2.31) |
| Daptomycin | 6 (1.3) | 4 (0.4) | 0.071 | 3.01 (0.86, 10.6) |
| Linezolid | 26 (5.4) | 23 (2.4) | 0.003 | 2.39 (1.34, 4.29) |
| Rifampicin | 10 (2.1) | 6 (0.6) | 0.013 | 3.41 (1.23, 9.45) |
| Carbapenem | 152 (31.6) | 83 (8.6) | <0.001 | 4.85 (3.60, 6.53) |
| Polymyxin | 20 (4.2) | 15 (1.6) | 0.002 | 2.80 (1.41, 5.57) |
| Antibiotic use (by cumulative number of agents), n (%)b | ||||
| None | 63 (13.1) | 370 (38.5) | <0.001 | 0.23 (0.17, 0.31) |
| 1 | 74 (15.4) | 272 (28.3) | <0.001 | 0.46 (0.34, 0.61) |
| 2 | 138 (28.7) | 164 (17.0) | <0.001 | 1.95 (1.50, 2.52) |
| ≥3 | 206 (42.8) | 156 (16.2) | <0.001 | 3.87 (3.00, 4.98) |
| Total antibiotic therapy duration, mean (SD), days | 30.2 (41.7) | 19.0 (28.4) | <0.001 | |
| Total duration of antibiotic therapy ≥15 days, n (%) | 262 (54.5) | 235 (24.4) | <0.001 | 3.78 (2.98, 4.80) |
| Acid suppressant use, n (%) | ||||
| Any | 387 (80.5) | 675 (70.2) | <0.001 | 2.19 (1.60, 3.00) |
| H2-receptor antagonist | 54 (11.2) | 95 (9.9) | 0.356 | 1.21 (0.80, 1.82) |
| Proton pump inhibitor | 360 (74.8) | 619 (64.3) | <0.001 | 1.70 (1.32, 2.20) |
| Total duration of acid suppressive therapy ≥15 days, n (%) | 253 (52.6) | 229 (23.8) | <0.001 | 3.82 (2.99, 4.88) |
| Immunosuppressant agent use, n (%) | ||||
| Any | 143 (29.7) | 208 (21.6) | <0.001 | 1.64 (1.26, 2.14) |
| Chemotherapy | 42 (8.7) | 49 (5.1) | 0.006 | 1.83 (1.18, 2.82) |
| Corticosteroid | 127 (26.4) | 177 (18.4) | <0.001 | 1.63 (1.25, 2.13) |
| Calcineurin inhibitor | 23 (4.8) | 34 (3.5) | 0.230 | 1.41 (0.80, 2.49) |
| Lactulose use, n (%) | 56 (11.6) | 106 (11.0) | 0.638 | 1.09 (0.76, 1.55) |
| Enteral feeding, n (%) | 91 (18.9) | 81 (8.4) | <0.001 | 2.91 (2.05, 4.11) |
OR = odds ratio; SD = standard deviation.
Antibiotic therapy duration in the case group was longer than in the control group (mean of total therapy duration, 30.2 vs 19.0 days, respectively; p < 0.001), and 54.5% (262/481) of cases compared with only 24.4% (235/962) of patients in the control group had antibiotic treatment periods of ≥15 days (p < 0.001; Table 3). Use of acid suppressants, use of immunosuppressants, and enteral feeding were observed more frequently among cases than in the control group.
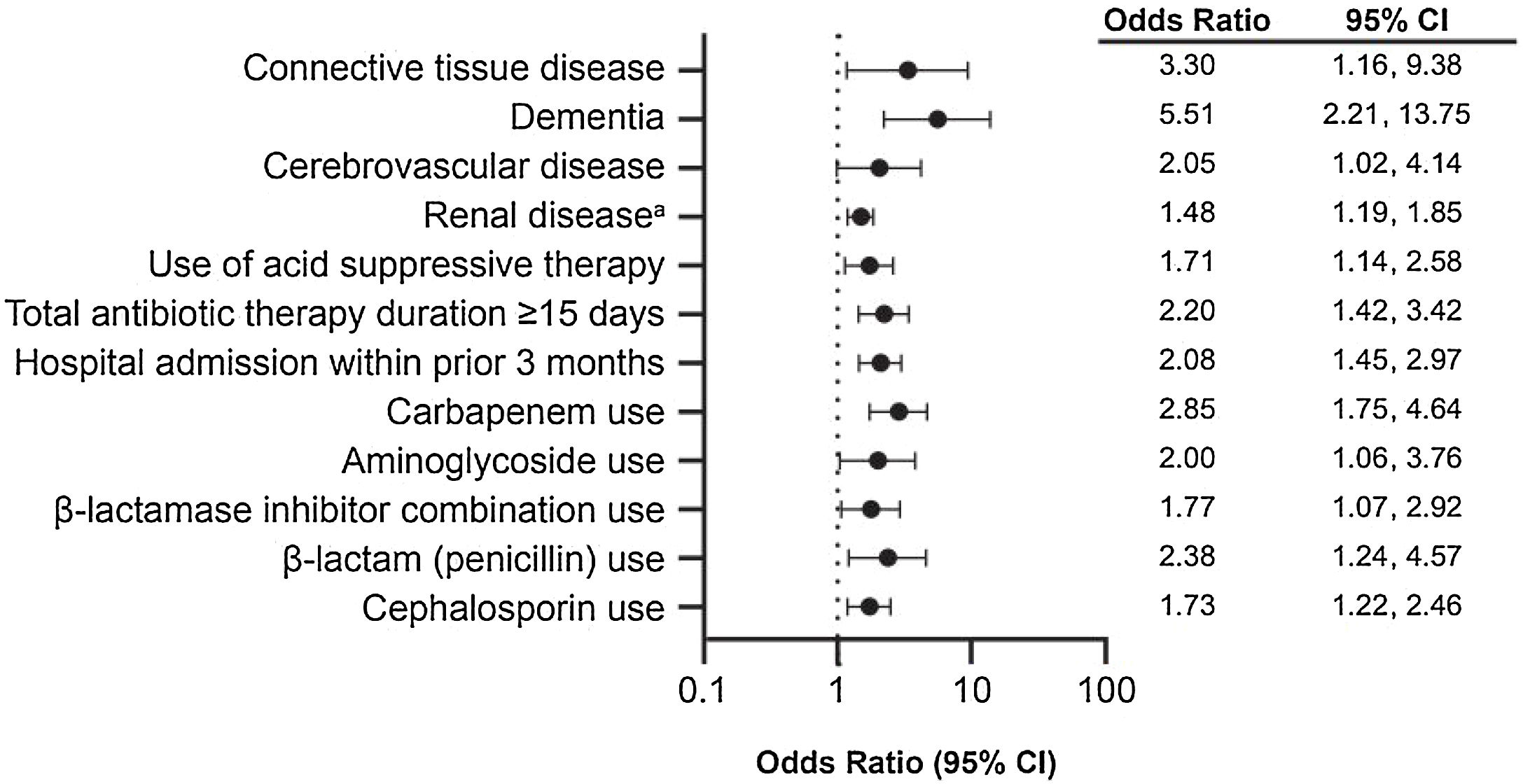
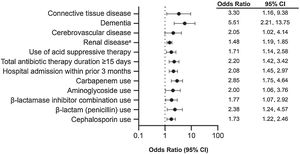
Multivariate analyses adjusting for comorbidities (AIDS, congestive heart failure, diabetes, connective tissue disease, dementia, diverticular disease, hemiplegia, inflammatory bowel disease, leukemia, cerebrovascular disease, renal disease, peripheral vascular disease, recent major surgery, and CCI > 3); hospital factors (intensive care unit [ICU] admission and admission within previous three months); use of acid suppressants, immunosuppressants, enteral feeding, and antibiotics (carbapenem, cephalosporin, aminoglycoside, beta-lactamase inhibitor combination, clindamycin, fluoroquinolone, penicillin, sulfonamide, tetracycline, daptomycin, linezolid, rifampicin, and polymyxin); and antibiotic therapy duration ≥15 days were further analyzed to identify risk factors associated with CDI in the study population (Fig. 3). In the fitted model, the effects of carbapenem use, comorbid moderate or severe renal disease, hospital admission within the prior three months, comorbid dementia, and total antibiotic therapy duration ≥15 days were significantly associated with CDI diagnosis after adjusting for other covariates. Specifically, the ORs of developing CDI for a patient with comorbid dementia and moderate or severe renal disease were 5.51 and 1.48, respectively. The OR of developing CDI for a patient previously admitted to the hospital in the past three months was 2.08 compared with someone who had not been hospitalized. Finally, the OR of developing CDI for a person who received carbapenem was 2.85.
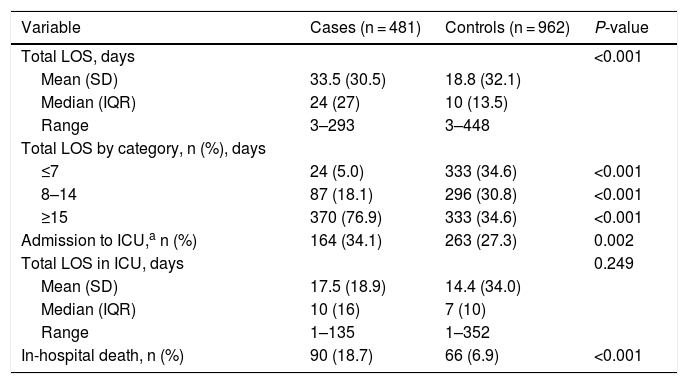
C. difficile infection burdenThe case group showed a longer mean overall LOS (33.5 days) than the control group (18.8 days), respectively (p < 0.001) and a longer mean ICU LOS (17.5 vs 14.4 days, respectively; p = 0.249; Table 4). Additionally, the case group presented a mean excess of overall LOS of 14.5 days (95% CI: 11.9–17.1 days) and an excess in ICU LOS of 3.7 days (95% CI: –2.1–9.6 days) attributable to CDI compared with the control group (not shown in Table 4).
Burden of CDI, Including LOS and In-Hospital Mortality.
| Variable | Cases (n = 481) | Controls (n = 962) | P-value |
|---|---|---|---|
| Total LOS, days | <0.001 | ||
| Mean (SD) | 33.5 (30.5) | 18.8 (32.1) | |
| Median (IQR) | 24 (27) | 10 (13.5) | |
| Range | 3–293 | 3–448 | |
| Total LOS by category, n (%), days | |||
| ≤7 | 24 (5.0) | 333 (34.6) | <0.001 |
| 8–14 | 87 (18.1) | 296 (30.8) | <0.001 |
| ≥15 | 370 (76.9) | 333 (34.6) | <0.001 |
| Admission to ICU,a n (%) | 164 (34.1) | 263 (27.3) | 0.002 |
| Total LOS in ICU, days | 0.249 | ||
| Mean (SD) | 17.5 (18.9) | 14.4 (34.0) | |
| Median (IQR) | 10 (16) | 7 (10) | |
| Range | 1–135 | 1–352 | |
| In-hospital death, n (%) | 90 (18.7) | 66 (6.9) | <0.001 |
CDI = Clostridioides difficile infection; ICU = intensive care unit; IQR = interquartile range; LOS = length of hospital stay; SD = standard deviation.
Overall, 156 deaths (10.8%) occurred during hospitalization. The in-hospital mortality rate was significantly greater among the case group compared to the control group (18.7% vs 6.9%, respectively; p < 0.001) (Table 4). The OR for an in-hospital death in a patient with CDI versus one without CDI was 3.23 (95% CI: 2.29, 4.55; not shown in Table 4). No differences were observed between patients in the case and control groups with an in-hospital death in terms of age, age group, and sex.
Multivariate analysis of cases versus controls found nosocomial CDI (OR 2.60; 95% CI: 1.75–3.85) and total LOS ≥ 15 days (OR 1.64; 95% CI: 1.09–2.47) to be major predictors of in-hospital mortality. In a separate multivariate analysis among patients in the case group, treatment failure (OR 22.05; 95% CI: 10.29–47.27) and severity of CDI as measured with the ATLAS score (OR 1.45 per each 1-unit increase on severity; 95% CI: 1.23–1.72) were major predictors of in-hospital mortality.
C. difficile infection management and outcomesAmong the 481 patients in the case group, the mean interval between hospital admission and onset of nosocomial diarrhea was 15.0 days (SD 16.0; 95% CI: 13.4–16.3); the mean total duration of diarrhea as noted in the medical record was 8.5 days (SD 7.1; 95% CI: 7.9–9.2). Surgical therapy for CDI was required in 1.1% (5/469) of patients. Concomitant therapy with probiotics was prescribed for 17.1% (77/451) of patients.
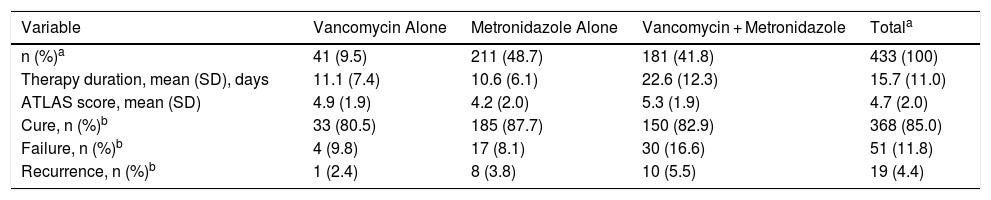
Of the 433 patients in the case group with outcome and antibiotic therapy data, metronidazole was the most commonly prescribed drug (90.5%; 392/433); combined use of metronidazole with vancomycin was reported in 41.8% (181/433) of patients (Table 5).
Antibiotic Treatments for CDI Among Cases With Antibiotic Information (n = 433).
| Variable | Vancomycin Alone | Metronidazole Alone | Vancomycin + Metronidazole | Totala |
|---|---|---|---|---|
| n (%)a | 41 (9.5) | 211 (48.7) | 181 (41.8) | 433 (100) |
| Therapy duration, mean (SD), days | 11.1 (7.4) | 10.6 (6.1) | 22.6 (12.3) | 15.7 (11.0) |
| ATLAS score, mean (SD) | 4.9 (1.9) | 4.2 (2.0) | 5.3 (1.9) | 4.7 (2.0) |
| Cure, n (%)b | 33 (80.5) | 185 (87.7) | 150 (82.9) | 368 (85.0) |
| Failure, n (%)b | 4 (9.8) | 17 (8.1) | 30 (16.6) | 51 (11.8) |
| Recurrence, n (%)b | 1 (2.4) | 8 (3.8) | 10 (5.5) | 19 (4.4) |
CDI = Clostridioides difficile infection; SD = standard deviation.
There were 85.0% (368/433) of patients who were considered to be cured of the CDI infection (Table 5). Treatment failure was observed in 11.8% (51/433) of patients, and CDI recurred in 4.4% (19/433) of patients. Of the antibiotics assessed, patients treated with metronidazole alone had the highest rate of cure; however, the mean ATLAS score was lower in this subset, indicating lower severity of CDI.
DiscussionThis hospital-based, case-control assessment conducted in Argentina, Brazil, Chile, and Mexico identified antibiotic exposure, existing medical conditions, and recent hospital admission as major risk factors for healthcare-associated CDI. These risk factors are consistent with those identified in Western and Latin American populations as described in this discussion.
Antibiotic exposure is thought to increase the risk of CDI via alterations of the normal intestinal flora, leading to overgrowth or new infection of C. difficile species.3 This risk is greatest at the start of antibiotic initiation but can persist for several months after discontinuation of the antimicrobial agent. Antibiotic exposure was identified as a major risk factor for healthcare-associated CDI in the current study, with use of ≥3 antibiotics and ≥15-day duration of antibiotic treatment having the strongest associations with CDI occurrence. These findings are comparable to those from Western and Latin American data sets. For instance, a US retrospective cohort study found that this risk appears to increase with a greater cumulative number of doses, number of antibiotics used, and duration of antimicrobial therapy; these factors were postulated by the authors to increase depletion of the normal gut flora, conferring greater risk of CDI development.31 Additionally, in a case-control study conducted at a tertiary hospital in Mexico, previous antibiotic use was more frequent (95.9% vs 79.9%; p < 0.001) and the number of antibiotics used was higher (3 vs 2; p < 0.001) in cases versus controls, respectively.32 Similarly, another case-control study conducted at a tertiary care hospital in Mexico found that prior antibiotic exposure was the most significant risk factor associated with CDI.33 A systematic review of studies in Brazil from 1988 to 2018 also concluded that the major risk factors for CDI were the number of previous antibiotics and duration of therapy.34
Use of high-risk antibiotics (clindamycin, carbapenem, fluoroquinolone, or any cephalosporin) also showed a strong association with CDI in this study. These findings are comparable to those from Western and Latin American data sets. In a US study, receipt of certain antibiotics, including cephalosporins and fluoroquinolones, resulted in greater risk of developing CDI.31 Additionally, multivariate analyses of a prospective cross-sectional study of three tertiary care hospitals in Colombia found exposure to third-generation cephalosporins to be a significant risk factor for CDI.35 In a prospective surveillance study of hospitals in Argentina and Mexico, several antibiotics were used significantly more among laboratory-confirmed cases of CDI compared with patients without CDI, including clindamycin and carbapenem.28 Similarly, hospital-based studies from Brazil and Mexico found a significant association with development of CDI and carbapenem use.32,36 Conversely, a study of eight university hospitals in Brazil found exposure to fluoroquinolones to be the only variable associated with development of CDI, whereas carbapenem exposure was not significantly associated.37
Patients with more comorbid conditions and with specific comorbidities are more susceptible to CDI.3 For instance, pre-existing inflammatory bowel disease is associated with increased incidence and severity of CDI.38 Other comorbidities demonstrably associated with CDI include chronic liver and kidney disease.3 In the current study, a CCI > 3, indicative of a greater number of comorbidities, was one of the factors most strongly associated with CDI occurrence. Additionally, multivariate analyses found substantially increased odds of developing CDI in patients with comorbid dementia or moderate/severe renal disease compared with patients who did not have these pre-existing conditions. Consistent with these findings, a hospital-based study in Brazil found that comorbidity severity, as measured by CCI at the time of hospital admission, was a strong independent predictor of CDI-associated diarrhea.36 Similarly, a US retrospective cohort study of healthcare-associated CDI found that patients with a greater number of comorbidities had a greater risk of CDI, even after controlling for potentially confounding variables, including antibiotic therapy and age.39 In a case-control study conducted at a tertiary care hospital in Mexico, patients with chronic kidney disease were the largest proportion of hospitalized patients with CDI.33
The current study found that the risk of developing CDI in patients with a recent hospitalization (within three months of current hospitalization) was more than two-fold higher compared to patients who had not been recently discharged from a hospital. This higher risk may reflect that patients with a recent hospitalization were sicker and with more comorbidities, thus more susceptible to CDI. Another factor for the increased risk in healthcare settings is associated with the more likely prevalence of C. difficile spores in locations such as hospitals and long-term care facilities.3 For instance, in a study of a tertiary hospital in Mexico, referral from another hospital resulted in a significant adjusted OR of 1.99 for development of hospital-onset, healthcare facility―associated CDI.32
Advanced age is a reported risk factor for CDI development and CDI-associated death, with the risk of developing CDI estimated to increase by 2% per year after 65 years of age.3,7 The reported high burden of CDI in older populations is multifactorial, with physiologic changes associated with ageing that may predispose elderly individuals to CDI (eg, immunosenescence and intestinal flora changes), frequent interaction with healthcare systems, increased use of antibiotics, and higher likelihood of comorbid conditions thought to be contributing factors.3,10 However, because no difference between the ages of cases and controls was observed in the current study, age as a risk factor for CDI could not be shown. Based on the preponderance of data from Western settings, future study of age-related effects on CDI risk in Latin America is warranted.
Our study found a considerable burden of disease in the study population; CDI was associated with increased in-hospital risk of death and longer LOS. Specifically, patients with CDI had a risk of dying in the hospital more than three-fold higher and an increased overall LOS of 14.5 days compared with patients without CDI. These findings are consistent with those in the existing literature showing that CDI can cause substantial morbidity and mortality, including within Latin America.1,11,27,32,40 For instance, in a hospital study from Colombia, the mortality rate from CDI was 13%.40 In a study of four Mexican hospitals, patients with a CDI diagnosis had a mortality rate of 9.0% and increased LOS of 15 days.27 Similarly, in a tertiary hospital in Mexico, LOS was 15 days longer in patients with CDI versus controls (25 vs 10 days, respectively; p < 0.001).32
The findings from this study indicate that CDI management in Latin America is complex, and unfavorable outcomes are frequent. All patients with CDI were treated with metronidazole, alone or combined with vancomycin, the most common antibiotic regimens. Although the majority of patients were cured of their infection, 16% experienced either treatment failure or recurrence during the same hospitalization. Consistent with this study, a publication of data from four Mexican hospitals found that 48% of patients with CDI were initially treated with vancomycin combined with metronidazole and 35% with metronidazole alone.40 Available guidance from Latin America recommends the use of vancomycin, with metronidazole considered as an alternative therapy.41–43 Notably, the choice of therapy varies by recommending body according to severity of CDI and availability of the various antimicrobials. The current study also showed that metronidazole was used more often among patients with less severe CDI; more severe cases tended to be treated with vancomycin alone or in combination with metronidazole. It is possible that the severity of CDI was more associated with treatment failure than attributed to the specific antibiotic regimen used. However, because the study was not powered to assess associations between type of antibiotic therapy and outcomes, it is not possible to determine the effect the common use of metronidazole-based regimens had on patient outcomes.
The strengths of this study are the relatively large sample size and that, to the best of our knowledge, it is one of the few studies from Latin America assessing risk factors associated with nosocomial CDI or consequences of CDI, such as mortality. In addition, cases and controls were selected from the same population with a risk-set sampling approach applied for matching, thereby minimizing the risk of bias.
This study was limited by the use of hospital databases and medical records, where the clinical aspects might not be fully captured and thus may introduce bias, and incompleteness of data capture could vary between countries and study centers. The study was also limited by the availability of data in medical records (eg, lack of follow-up data on readmission at other healthcare facilities), which may have caused the estimation of CDI recurrence to be biased. The use of hospital databases and medical records also precluded assessment of some outcomes of interest, such as the prevalence of antibiotic resistance by treatment received and type of ribotype circulating. Additionally, because the study was only conducted in four Latin American countries, generalizability to other countries in the region may be limited because of differences in diagnostic and treatment practices. For instance, testing for anaerobic pathogens, such as C. difficile, is not routine in many laboratories in Latin America.17 Accordingly, because underdiagnosis of CDI is problematic in Latin America, misclassification of cases might occur. However, this was addressed in the study in that patients with diarrhea were excluded from the control group, thereby avoiding misclassification.
In conclusion, this study found that antibiotic exposure, existing medical conditions, and recent hospital admission were major risk factors for healthcare-associated CDI in select countries in Latin America. Because of the limited data available on CDI epidemiology and risk factors within this region, these findings further emphasize the importance of improved surveillance to better characterize and understand the burden of CDI in Latin America. Such information is critical to identify and implement effective prevention and infection control measures.
Declaration of interestH Yu and D Curcio are employees of Pfizer Inc and hold stocks and stock options. N Flaster and A Casanello have no competing interests to declare.
The authors thank the investigators of this study, including Pablo Bonvehí (CEMIC, Argentina), Liliana Fernandez Canigia (Hospital Alemán, Argentina), Verónica Berdiñas (Hospital Bernardo Houssay, Argentina), Thais Guimarães (Instituto Central Hospital das Clínicas, Brazil), Maura Salaroli (Hospital Sírio Libanês, Brazil), Alejandra Marcotti (Clínica Alemana, Chile), Maria del Rayo Morfín Otero (Hospital Civil de Guadalajara “Fray Antonio Alcalde,” Mexico), and Adrián Camacho (Hospital Universitario “Dr. José Eleuterio González,” Mexico). Editorial/medical writing support was provided by Tricia Newell, PhD, of ICON plc (North Wales, PA), and was funded by Pfizer Inc.