To evaluate the virological outcomes in children and adolescents infected with HIV-1 in Salvador, Bahia according to genotyping results.
MethodsWe retrospectively evaluated the rates of virological suppression of children and adolescents submitted to HIV-1 genotyping test from January/2008 to December/2012. The participants were followed in the two referral centers for pediatric AIDS care, in Salvador, Brazil. Resistance mutations, drug sensitivity profiles, and viral subtypes were analyzed using the Stanford HIV-1 Drug Resistance Database. Adherence was estimated by drugs withdrawal at pharmacies of the two sites.
Results101 subjects were included: 35 (34.6%) were drug-naïve, and the remaining 66 were failing ART. In drug-naïve group, 3 (8.6%), presented with NNRTIs resistance mutations, along with polymorphic mutations to PIs in most (82.8%) of them. Among the failing therapy group, we detected a high frequency (89.4%) of resistance mutations to PIs, NRTI (84.8%), and NNRTI (59.1%). Virological suppression after introduction/modification of genotyping-guided ART was achieved only for patients (53.1%) with drug withdrawal over 95%. Main detected HIV-1 subtypes were B (67.3%), F (7.9), C (1.9%), and recombinant forms (22.9%).
ConclusionsDespite the use of genotyping tests in guidance of a more effective antiretroviral regimen, poor adherence to ART seems to be the main determinant of low virological suppression rate for children and adolescents, in Salvador, Brazil.
In the last decade, AIDS epidemic has increased among women under reproductive age worldwide, increasing the number of at risk children to mother-to-child transmission (MTCT) of HIV-1. In Brazil, 798,366 cases of AIDS were reported between 1980 and 2015, and about 39,554 correspond to children and adolescents less than 18 years of age. In 92.6% of those younger than 13 years of age, infection occurred through MTCT.1,2
The introduction of highly active antiretroviral therapy (HAART) significantly reduced the epidemic spread in pediatric population, as well as the mortality and morbidity associated with HIV-1 infection.3–5 However, it requires adequate (>95%) adherence levels to therapy to prevent or reduce the risk of virological rebound and emergence of resistant strains.6–8 Some challenges limit the adherence in children and the efficacy of the ARV therapy: complex antiretroviral regimens, side effects, low availability of pediatric formulations, lifelong duration of therapy, and dependence on a caregiver to administrate the medication.9–11
HIV-1 resistance to ARV drugs is a consequence of the occurrence of mutations in the viral genome.12–14 The Brazilian Ministry of Health/National HIV-1/AIDS Program, since 2009 provides free resistance testing for children starting HAART or failing a previous treatment regimen.15 Viral diversity also plays an important role in the response to antiretroviral treatment. Some studies have demonstrated specific subtype differences in HIV-1 susceptibility to ARV drugs and the identification of mutations selected by ART,16–24 but the relationship between viral diversity and therapeutic response in children and adolescents is still poorly understood.
The goal of this work was to evaluate the factors driving the response to ARV therapy in children and adolescents infected with HIV-1 in Salvador-Bahia, and the impact of HIV-1 genotyping test on virological outcomes for this population.
Materials and methodsPopulation and study designThis retrospective cross-sectional study was conducted at the Infectious Diseases Research Laboratory (LAPI), located at Professor Edgard Santos University Hospital (HUPES) in Salvador/Bahia. All genotyping assays performed from January/2008 to December/2012 for failing or drug-naïve patients under 18 years of age, from Salvador/Ba, were reviewed. These subjects were under regular care at HUPES or at Specialized State Center for Diagnosis, Care and Research (CEDAP). We excluded patients without available laboratory information in a period of at least six months after ART introduction or switching. The Institutional Review Committee approved the study.
Demographic, clinical and laboratory dataWe collected clinical and demographic information from existing electronic databases. Pre and post (≥6 months) therapy HIV-1 plasma viral load (VL) were recorded, to evaluate the virological response after ART introduction/switching. Adequate virological response was defined as less than 50copies/ml, at least six months after initiation of genotyping guided ART.
Genotyping assay and HIV-1 subtypingThe genotyping assay was performed using a commercially available Kit (TRUGENE HIV-1 Genotyping Kit Siemens/Bayer Co. USA), according to manufacturer's instructions. Interpretation of resistance test was performed according to Stanford HIV-1 resistance database (http://hvdb.stanford.edu). An infectious diseases specialist, member of the Brazilian Ministry of Health's advisory group for interpretation of genotypic tests (the so-called reference physician on genotyping interpretation – RPG) provided recommendations on the best antiretroviral regimen to treat the patients submitted to genotyping.
Adherence to therapyTreatment adherence was assessed by drug withdrawal from pharmacy. Optimal adherence was defined as acquisition of ARV drugs in ≥95% of times they were prescribed, and when there were less than eight days delay for refilling the monthly quota of ARVs.
Statistical analysesSPSS (Statistical Package for Social Sciences) version 17.0 was used to perform all statistical calculations. Descriptive analyses (frequencies, median and interquartile range – IQR 25–75%) were performed, and comparisons between groups were assessed by chi-square test. Non-parametric tests were used to evaluate continuous variables.
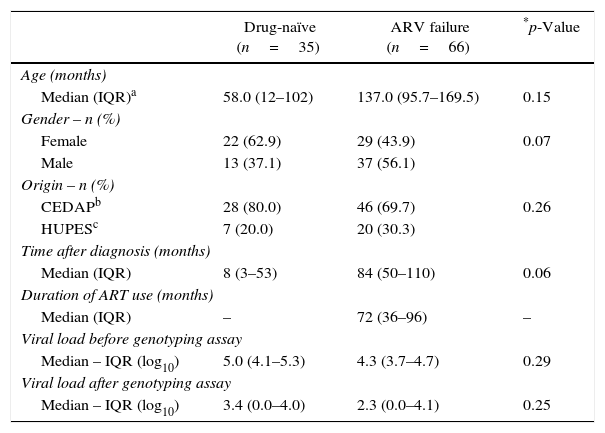
ResultsDuring the study period, 279 children and adolescents were submitted to genotyping assays in the state of Bahia, and of these, 121 (43.4%) corresponded to samples from Salvador city. Twenty subjects were excluded due to low VL (<1000copies/ml). Therefore, our total sample consisted in 101 subjects, 35 of whom were drug-naïve and 66 were failing ART. All subjects were infected with HIV-1 through vertical route. Table 1 shows the characteristics of the study population.
Characteristics of study population.
| Drug-naïve (n=35) | ARV failure (n=66) | *p-Value | |
|---|---|---|---|
| Age (months) | |||
| Median (IQR)a | 58.0 (12–102) | 137.0 (95.7–169.5) | 0.15 |
| Gender – n (%) | |||
| Female | 22 (62.9) | 29 (43.9) | 0.07 |
| Male | 13 (37.1) | 37 (56.1) | |
| Origin – n (%) | |||
| CEDAPb | 28 (80.0) | 46 (69.7) | 0.26 |
| HUPESc | 7 (20.0) | 20 (30.3) | |
| Time after diagnosis (months) | |||
| Median (IQR) | 8 (3–53) | 84 (50–110) | 0.06 |
| Duration of ART use (months) | |||
| Median (IQR) | – | 72 (36–96) | – |
| Viral load before genotyping assay | |||
| Median – IQR (log10) | 5.0 (4.1–5.3) | 4.3 (3.7–4.7) | 0.29 |
| Viral load after genotyping assay | |||
| Median – IQR (log10) | 3.4 (0.0–4.0) | 2.3 (0.0–4.1) | 0.25 |
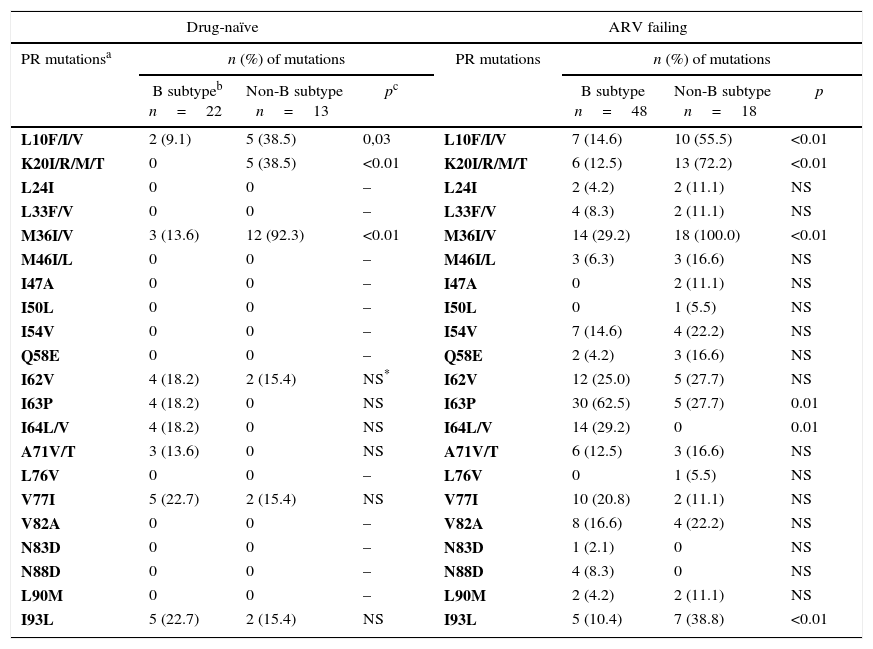
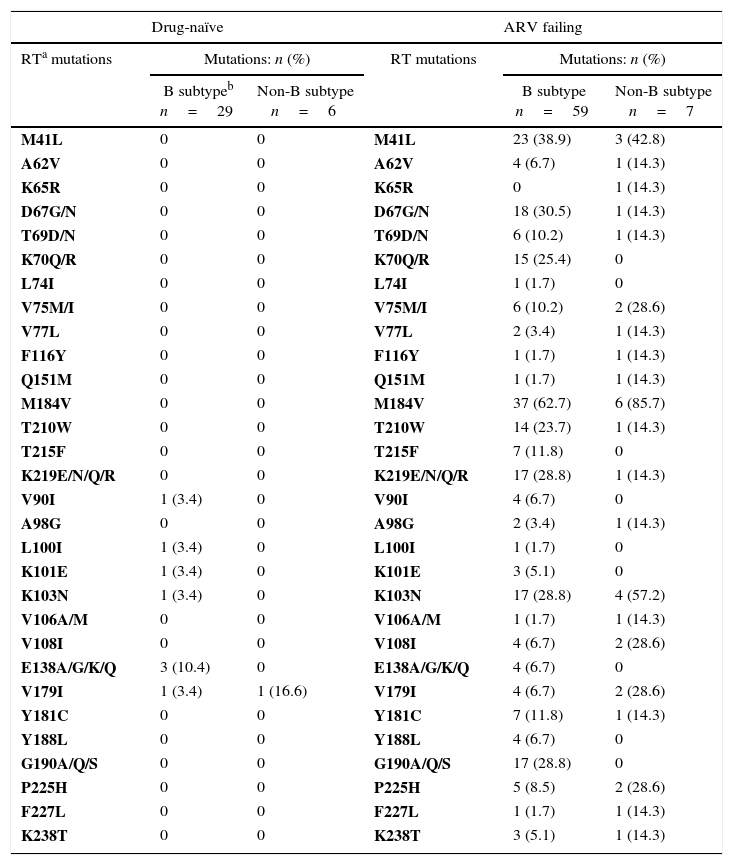
In drug-naïve group, we detected only secondary mutations in HIV-1 protease region (Table 2). Primary resistance mutations (L100I, K101E and K103N), associated to non-nucleoside reverse transcriptase (NNRTI) drugs were detected in three subjects (8.6%) (Table 3). However, in ARV failing group, as expected, we detected a more complex pattern of drug-resistance mutations, including amino acid changes associated with resistance to nucleoside reverse transcriptase inhibitors (NRTIs), NNRTIs, and PI, as shown in Tables 2 and 3.
Association of antiretroviral resistance mutations in the PR regiona according to HIV-1 subtypes in naïve and ARV failing children and adolescents.
| Drug-naïve | ARV failing | ||||||
|---|---|---|---|---|---|---|---|
| PR mutationsa | n (%) of mutations | PR mutations | n (%) of mutations | ||||
| B subtypeb n=22 | Non-B subtype n=13 | pc | B subtype n=48 | Non-B subtype n=18 | p | ||
| L10F/I/V | 2 (9.1) | 5 (38.5) | 0,03 | L10F/I/V | 7 (14.6) | 10 (55.5) | <0.01 |
| K20I/R/M/T | 0 | 5 (38.5) | <0.01 | K20I/R/M/T | 6 (12.5) | 13 (72.2) | <0.01 |
| L24I | 0 | 0 | – | L24I | 2 (4.2) | 2 (11.1) | NS |
| L33F/V | 0 | 0 | – | L33F/V | 4 (8.3) | 2 (11.1) | NS |
| M36I/V | 3 (13.6) | 12 (92.3) | <0.01 | M36I/V | 14 (29.2) | 18 (100.0) | <0.01 |
| M46I/L | 0 | 0 | – | M46I/L | 3 (6.3) | 3 (16.6) | NS |
| I47A | 0 | 0 | – | I47A | 0 | 2 (11.1) | NS |
| I50L | 0 | 0 | – | I50L | 0 | 1 (5.5) | NS |
| I54V | 0 | 0 | – | I54V | 7 (14.6) | 4 (22.2) | NS |
| Q58E | 0 | 0 | – | Q58E | 2 (4.2) | 3 (16.6) | NS |
| I62V | 4 (18.2) | 2 (15.4) | NS* | I62V | 12 (25.0) | 5 (27.7) | NS |
| I63P | 4 (18.2) | 0 | NS | I63P | 30 (62.5) | 5 (27.7) | 0.01 |
| I64L/V | 4 (18.2) | 0 | NS | I64L/V | 14 (29.2) | 0 | 0.01 |
| A71V/T | 3 (13.6) | 0 | NS | A71V/T | 6 (12.5) | 3 (16.6) | NS |
| L76V | 0 | 0 | – | L76V | 0 | 1 (5.5) | NS |
| V77I | 5 (22.7) | 2 (15.4) | NS | V77I | 10 (20.8) | 2 (11.1) | NS |
| V82A | 0 | 0 | – | V82A | 8 (16.6) | 4 (22.2) | NS |
| N83D | 0 | 0 | – | N83D | 1 (2.1) | 0 | NS |
| N88D | 0 | 0 | – | N88D | 4 (8.3) | 0 | NS |
| L90M | 0 | 0 | – | L90M | 2 (4.2) | 2 (11.1) | NS |
| I93L | 5 (22.7) | 2 (15.4) | NS | I93L | 5 (10.4) | 7 (38.8) | <0.01 |
Association of ARV resistance mutations in the RTa region according to HIV-1 subtypes in drug-naïve and ARV failing in children and adolescents.
| Drug-naïve | ARV failing | ||||
|---|---|---|---|---|---|
| RTa mutations | Mutations: n (%) | RT mutations | Mutations: n (%) | ||
| B subtypeb n=29 | Non-B subtype n=6 | B subtype n=59 | Non-B subtype n=7 | ||
| M41L | 0 | 0 | M41L | 23 (38.9) | 3 (42.8) |
| A62V | 0 | 0 | A62V | 4 (6.7) | 1 (14.3) |
| K65R | 0 | 0 | K65R | 0 | 1 (14.3) |
| D67G/N | 0 | 0 | D67G/N | 18 (30.5) | 1 (14.3) |
| T69D/N | 0 | 0 | T69D/N | 6 (10.2) | 1 (14.3) |
| K70Q/R | 0 | 0 | K70Q/R | 15 (25.4) | 0 |
| L74I | 0 | 0 | L74I | 1 (1.7) | 0 |
| V75M/I | 0 | 0 | V75M/I | 6 (10.2) | 2 (28.6) |
| V77L | 0 | 0 | V77L | 2 (3.4) | 1 (14.3) |
| F116Y | 0 | 0 | F116Y | 1 (1.7) | 1 (14.3) |
| Q151M | 0 | 0 | Q151M | 1 (1.7) | 1 (14.3) |
| M184V | 0 | 0 | M184V | 37 (62.7) | 6 (85.7) |
| T210W | 0 | 0 | T210W | 14 (23.7) | 1 (14.3) |
| T215F | 0 | 0 | T215F | 7 (11.8) | 0 |
| K219E/N/Q/R | 0 | 0 | K219E/N/Q/R | 17 (28.8) | 1 (14.3) |
| V90I | 1 (3.4) | 0 | V90I | 4 (6.7) | 0 |
| A98G | 0 | 0 | A98G | 2 (3.4) | 1 (14.3) |
| L100I | 1 (3.4) | 0 | L100I | 1 (1.7) | 0 |
| K101E | 1 (3.4) | 0 | K101E | 3 (5.1) | 0 |
| K103N | 1 (3.4) | 0 | K103N | 17 (28.8) | 4 (57.2) |
| V106A/M | 0 | 0 | V106A/M | 1 (1.7) | 1 (14.3) |
| V108I | 0 | 0 | V108I | 4 (6.7) | 2 (28.6) |
| E138A/G/K/Q | 3 (10.4) | 0 | E138A/G/K/Q | 4 (6.7) | 0 |
| V179I | 1 (3.4) | 1 (16.6) | V179I | 4 (6.7) | 2 (28.6) |
| Y181C | 0 | 0 | Y181C | 7 (11.8) | 1 (14.3) |
| Y188L | 0 | 0 | Y188L | 4 (6.7) | 0 |
| G190A/Q/S | 0 | 0 | G190A/Q/S | 17 (28.8) | 0 |
| P225H | 0 | 0 | P225H | 5 (8.5) | 2 (28.6) |
| F227L | 0 | 0 | F227L | 1 (1.7) | 1 (14.3) |
| K238T | 0 | 0 | K238T | 3 (5.1) | 1 (14.3) |
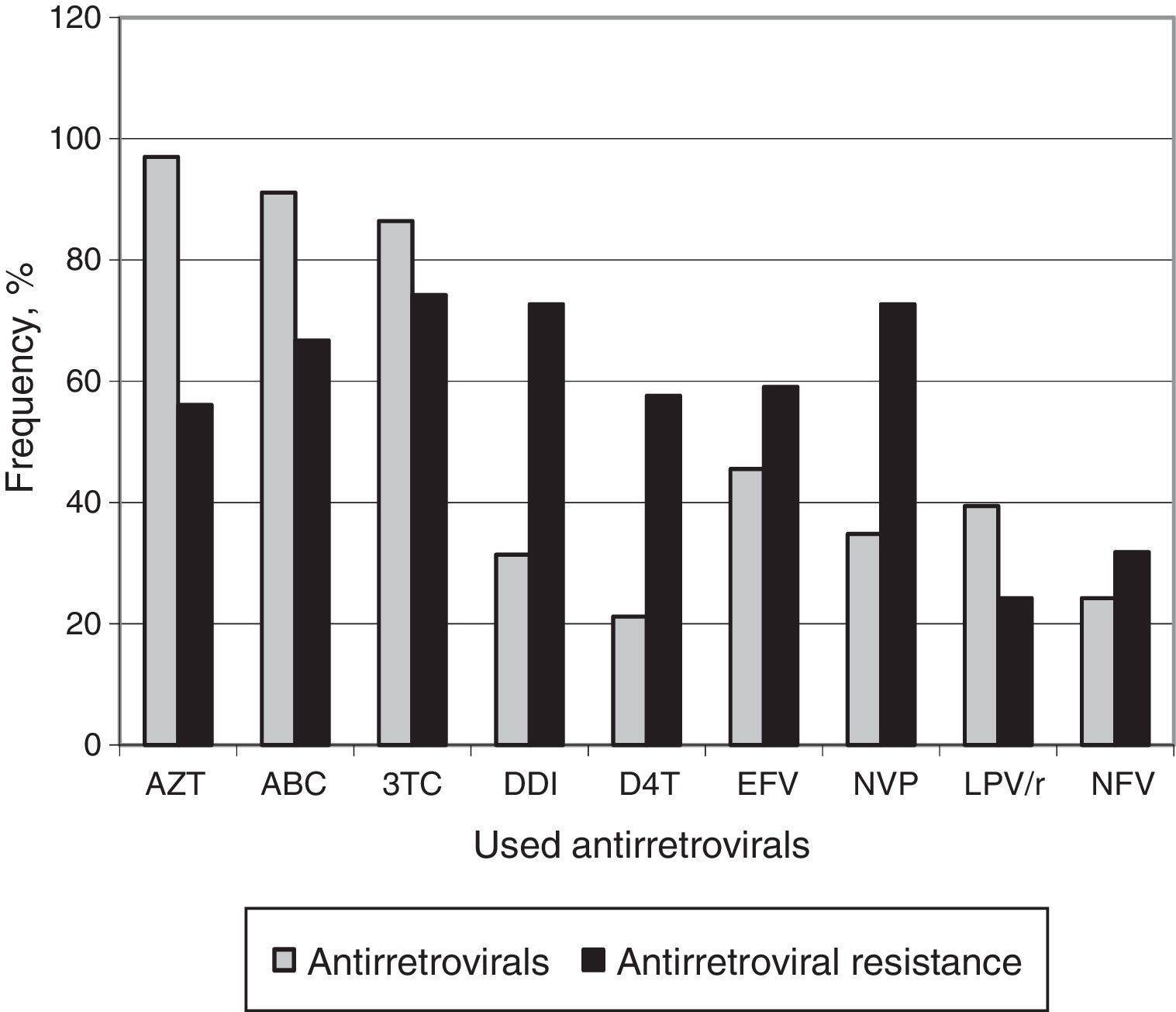
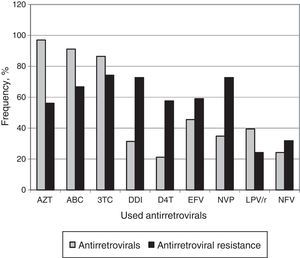
Among patients failing ART, AZT (97.0%) was the most previously used ARV drug. All subjects had been exposed to NRTIs, 45 (68.2%) to NNRTIs and 34 (51.5%) to PIs. Fig. 1 displays the frequency of ARV use and the resistance rates to these drugs.
After genotyping test, all ARV regimens proposed by the RPG for the 29 drug-naïve subjects starting ART, contained a genotypic sensitivity score (GSS) of three active drugs. In contrast, the ARV failing group had a median of two active drugs in treatment proposed by the RPG. However, prescribing physicians maintained the ARV regimens suggested by the RPG for only 65.3% of the subjects (27 drug-naïve and 35 ARV failure groups). The most frequently observed ARV regimens are shown in Fig. 1. Raltegravir was included in ARV regimens for eight subjects, while Enfuvirtide was prescribed for only two subjects. Six drug-naïve subjects did not receive ARV prescription.
During the period of six months following ARV prescription, 18 (51.4%) subjects on initial therapy and 43 (65.2%) with subsequent ART after failure, attended regularly the monthly scheduled visits to pharmacy to refill their ARV drugs. Virologic suppression after six months was achieved by only 43/81 (53.1%) children and adolescents. In this group, 13/29 (44.8%) were initiating therapy and 31/66 (46.9%) ART failing group. It is noteworthy that among all subjects who reached VL below 50copies/ml after introduction/switch of ART, only 23/43 (53.4%) used the ARV regimen proposed by the RPG, but adherence (measured by drug withdrawal) was greater than 95%. In addition, 35/66 (53%) subjects in treatment-experienced group, who did not achieve undetectable viral load after genotyping-guided switch, used ART regimen containing a median of 1.5 (1–3) active drugs.
Among the 101 samples tested, 68 (67.4%) were classified as subtype B, 8 (7.9%) as subtype F, 2 (1.9%) as subtype C, and 23 (22.8%) as recombinant forms. The recombinant forms found for RT and PR regions, were AB (1), BF (2), DB (1), FB (16), KB (2), and CRF02_AG (1). The frequency of mutations associated with HIV-1 subtypes found in drug-naïve group and in ARV failing group are shown in Tables 2 and 3.
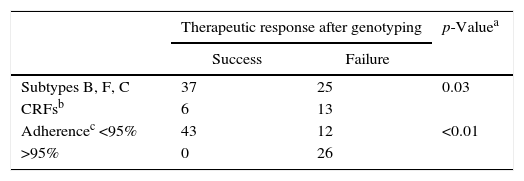
We observed different mutational frequency according to HIV-1 subtype: in PR region, L10F/I/V, K20I/R/M/T, M36I/V mutations were detected mainly in non-B subtypes. On the other hand, I63P or I64L/V were observed only in patients under ARV failure, and were more frequently detected among individuals harboring HIV-1 subtype B strain (p=0.01 and p=0.01, respectively, for comparison between B and non-B subtypes). The I93L mutation was also found only in ARV failure group, but it was more common among patients with non-B subtype. We did not detect any difference in frequency of NRTI/NNRTI mutations for different subtypes. In addition, there was no significant difference in the rates of response to therapy according to different subtypes (p=0.16). However, we found a significant difference when comparing pure subtypes and recombinant forms: patients infected with HIV-1 subtypes B, F or C, were more likely (52%) to achieve virological suppression in comparison with those infected by recombinant forms (27.3%, p=0.02). The difference remains significant when adjusting virological suppression rates for adherence levels (Table 4).
Association of HIV-1 subtypes and adherence to antiretroviral therapy with therapeutic response after genotyping in children naïve and with previous treatment failure.
| Therapeutic response after genotyping | p-Valuea | ||
|---|---|---|---|
| Success | Failure | ||
| Subtypes B, F, C | 37 | 25 | 0.03 |
| CRFsb | 6 | 13 | |
| Adherencec <95% | 43 | 12 | <0.01 |
| >95% | 0 | 26 | |
In this retrospective study, drug-naïve children presented with a high rate of secondary mutations associated with PI resistance, as already shown in previous works.20,21 The absence of mutations associated with NRTIs was unexpected, but similar to previous findings in Brazilian pediatric cohorts.17,21 Our data revealed a primary resistance rate of 17.2%, somewhat higher than that found by Vaz and cols. in 2015,25 but similar to the rate found by Pedroso and cols. in 2007,19 both studies conducted with HIV-1 infected children in Bahia. Late diagnosis of HIV-1 infection of mothers, lack of adequate prophylaxis against vertical transmission, low adherence and transmission of resistant viral strains are likely factors associated with this high rate of primary resistance.11,26,27
The most frequent mutations detected in our group reflect the use of ARV routinely prescribed for initial therapy. Lamivudine resistance mutation (M184V) was detected in 65.2% of patients, and, as expected, we observed a high frequency of Thymidine Analog Mutations (TAMs), due to the wide use of AZT as first-line therapy of children, in Brazil. The presence of these mutations affects the long-term efficacy of most NRTI-based therapeutic regimes.28 AZT (97%), ABC (91.1%), and 3TC (86.4%) were the most used drugs in previous ARV regimens, and this fact explains the detection of high resistance levels to these drugs.16,23,29
A striking feature of NNRTIs class is its low genetic barrier: the most commonly found mutations were K103N and G190A.14,30 These mutations are selected by EFV and NVP, which are indicated as first-line antiretroviral treatment by Brazilian ARV recommendations for the pediatric patients.14,15
In the present work, the prevalence of mutations associated with resistance in PI class, was similar to that found in previous studies.17,21,27,31 Although only LPV/r and NFV have been used in this population, we observed minor mutations conferring cross-resistance to all antiretroviral drugs in this class, which impairs their use as salvage therapy.27,32
Although studies conducted in adult cohorts demonstrate significant improvement in viral suppression after therapy guided by genotyping,30,33 in our study, only 43 (53.1%) subjects achieved VL<50 HIV-1 RNA plasma copies/ml. All patients who achieved virologic suppression (13/43 in initial therapy and 30/43 in salvage regimens) presented an adherence rate ≥95%. Among patients who did not achieve viral suppression, most of them showed irregular drug procurement. This is in accordance with previous studies that showed a close relationship between viral resistance, treatment failure, and irregular adherence.34–37 In addition, one main feature of HIV-1 infection in children is the high viral load they often present, which may need more time to reach viral suppression. In our study, however, the mean viral load was around 100,000copies/ml.
Regarding the strain diversity, the results confirm the prevalence of subtype B as the main HIV-1 variant circulating in Brazil, and the findings are in accordance with studies in adult and pediatric cohorts.16,21,23–25,27–29,38 The observed increase of non-B subtypes, such as the F and C, favors emergence of recombinant forms. Brazilian studies with children detected prevalence rates from 9.5 to 15% for subtype F,16,19,21,23,24,27–29 and 1.9 to 2.5% for subtype C,16,19 similar to our findings (7.9% F and 1.9% C). However, this is not the case in the South region in Brazil, where subtype C predominates, especially among pregnant women.21,23
Recombinant forms were detected in 22.8% of children and adolescents in our study, with predominance of BF and FB mosaics. Despite the frequent detection of BF mosaic at most studies,19,21,24,25,27 we found a higher frequency of FB mosaic (15.8%). Interestingly, children infected by recombinant forms in our study presented with a significantly lower rate of response to therapy. This suggests that resistance patterns might differ for these viral strains, making necessary to better define its specific mutational profile, and how it can affect response to therapy. The significant association between some resistance mutations and recombinant forms indicates that these mosaics may present a differential response to therapy, which can be driven by such mutations.
Although the frequency of primary resistance mutations in PR region, for subtypes B and non-B, was similar in naïve and treatment failing groups, we detected differences across subtypes when we analyzed secondary mutations at positions 10, 20, 36, 63, 64, and 93. Possibly, these variations are natural consequences of polymorphisms found in subtypes without any impact on therapeutic response, and these data coincide with previous studies.16,28,39,40 On the other hand, Kantor et al. in 2003 demonstrated that the increase in the number of accessory mutations and polymorphisms might result in different responses to therapy, when it occurs in non-B subtype.41 For instance, 20I mutation is the consensus amino acid in subtype G and CRF02_AG, but in subtypes B and C this mutation is selected by PIs and can reduce the susceptibility to NFV.40,42 Despite the difference in prevalence of K65R, other mutations followed similar patterns across subtypes B and non-B.
Virological and clinical studies suggest that differences between HIV-1 subtypes do not affect the response to therapy.41 However, this approach may be limited in identifying determinants of specific subtypes in the response to antiretroviral therapy, because there is no evidence that all non-B viruses behave in a similar way.41,42 Our study has some clear limitations: the retrospective design does not permit to obtain detailed data on adherence and other important variables in this kind of study. In addition, adherence was not directly measured, but the use of drug procurement, although not refined, is a direct evidence of adherence to therapy, since no drug possession means no drug intake.
We demonstrated that adherence to antiretroviral therapy is low among children and adolescents with HIV-1/AIDS, and are the main causes of therapeutic failure leading to the frequent detection of resistance mutations in the pediatric population. Taking into account the vulnerability of these patients, genotyping is useful to guide an appropriate and effective treatment. However, it may be useless if not coupled with enough active drugs prescribed for treating patients failing therapy. In addition, regular adherence to ART remains a decisive factor for therapeutic success.
Conflicts of interestThe authors declare no conflicts of interest.