To evaluate the HBeAg seroconversion rate in real clinical setting and explore its predictors in long-term nucleos(t)ide analogues (NAs) treatment for chronic hepatitis B (CHB).
Methods251 patients were recruited from January 2001 to September 2009 in four hospitals in Hebei province, China, for this retrospective study. Clinical and laboratory data before and after treatment with lamivudine (LAM, 100mg daily), adefovir (ADV, 10mg daily), telbivudine (LDT, 600mg daily), entecavir (ETV, 0.5mg daily), and LAM/ADV combination were compared among three groups according to treatment outcomes: synchronous HBeAg loss and HBeAg seroconversion, anti-HBe development after treatment, and no anti-HBe. Adherence was also evaluated.
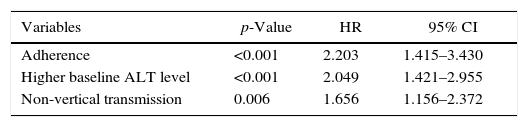
ResultsIn real clinical setting, cumulative HBeAg seroconversion rates were 14.3%, 32.7%, 43.0%, 46.9%, and 50.5% after 1, 2, 3, 5, and 8 years, respectively. 45 patients (17.9%) were non-adherent. Adherence (p<0.001, Hazard Ratio (HR)=2.203), elevated alanine aminotransferase (ALT) levels (p<0.001, HR=2.049), and non-vertical transmission (p=0.006, HR=1.656) were predictors of HBeAg seroconversion.
ConclusionAdherence, elevated ALT, and non-vertical transmission are predictors of HBeAg seroconversion in CHB patients treated with NAs.
Chronic hepatitis B (CHB) remains a major public health concern, because of worldwide prevalence and the potential to cause adverse consequences such as liver cirrhosis, hepatic decompensation, and hepatocellular carcinoma (HCC).1 However, CHB can be managed with effective antiviral agents. Hepatitis Be antigen (HBeAg) seroconversion is considered the treatment endpoint in HBeAg-positive patients.2 It is correlated with clinical remission accompanied by improved necro-inflammatory activity,3 fibrosis regression,4 lower risk of liver cirrhosis and HCC,5 and higher incidence of complication-free survival.6
Nucleos(t)ide analogues (NAs) are recommended as first-line anti-HBV agents owing to high antiviral potency, ease of use, and high tolerability in HBeAg-positive patients, especially those with high hepatitis B virus (HBV) DNA loads and liver cirrhosis. However, HBeAg seroconversion rates after treatment with five commonly prescribed oral antiviral agents are 12–23% after one year of treatment, 25–31% after two years, and up to 44% after three years according to recent clinical trials.7 In addition, two recent studies conducted in the United States revealed that HBeAg seroconversion rate is significantly lower in real clinical setting, especially in entecavir (ETV)-treated patients.8,9 HBeAg seroconversion rate in actual clinical setting was less than 10% during the first year of NAs therapy. A number of factors, such as older age, higher alanine aminotransferase (ALT) levels, lower HBV DNA load, genotype B (vs. C), and pathological factors, may predispose patients to HBeAg seroconversion either in the natural course or during individual NAs or interferon (IFN) therapies.10–13 However, few studies have evaluated HBeAg seroconversion rates during long-term NAs therapy in real clinical setting, but not assessing factors that may predict HBeAg seroconversion.
Currently, adherence studies mainly focus on anti-HIV therapies, and many options have been tested for adherence assessment, including visual analogue scale and the so-called 3-day recall instrument.14,15 Although such studies are less common in the HBV field, it was demonstrated that higher adherence to NAs treatment in CHB patients tended to result in decreased rate of viral breakthroughs.16
Thus, the goal of the current study was to evaluate long-term outcomes of NAs treatment through HBeAg seroconversion rates, whose predictive factors were also assessed, in a multicenter set of CHB treatment-naive patients in real clinic in China.
Materials and methodsStudy design and patient populationThis was a retrospective case control study of consecutive treatment-naïve CHB patients treated with lamivudine (LAM, 100mg daily), adefovir (ADV, 10mg daily), telbivudine (LDT, 600mg daily), ETV (0.5mg daily), and LAM and ADV combination for more than three years from January 2001 to September 2009 at five departments of gastroenterology and hepatology in Chinese hospitals.
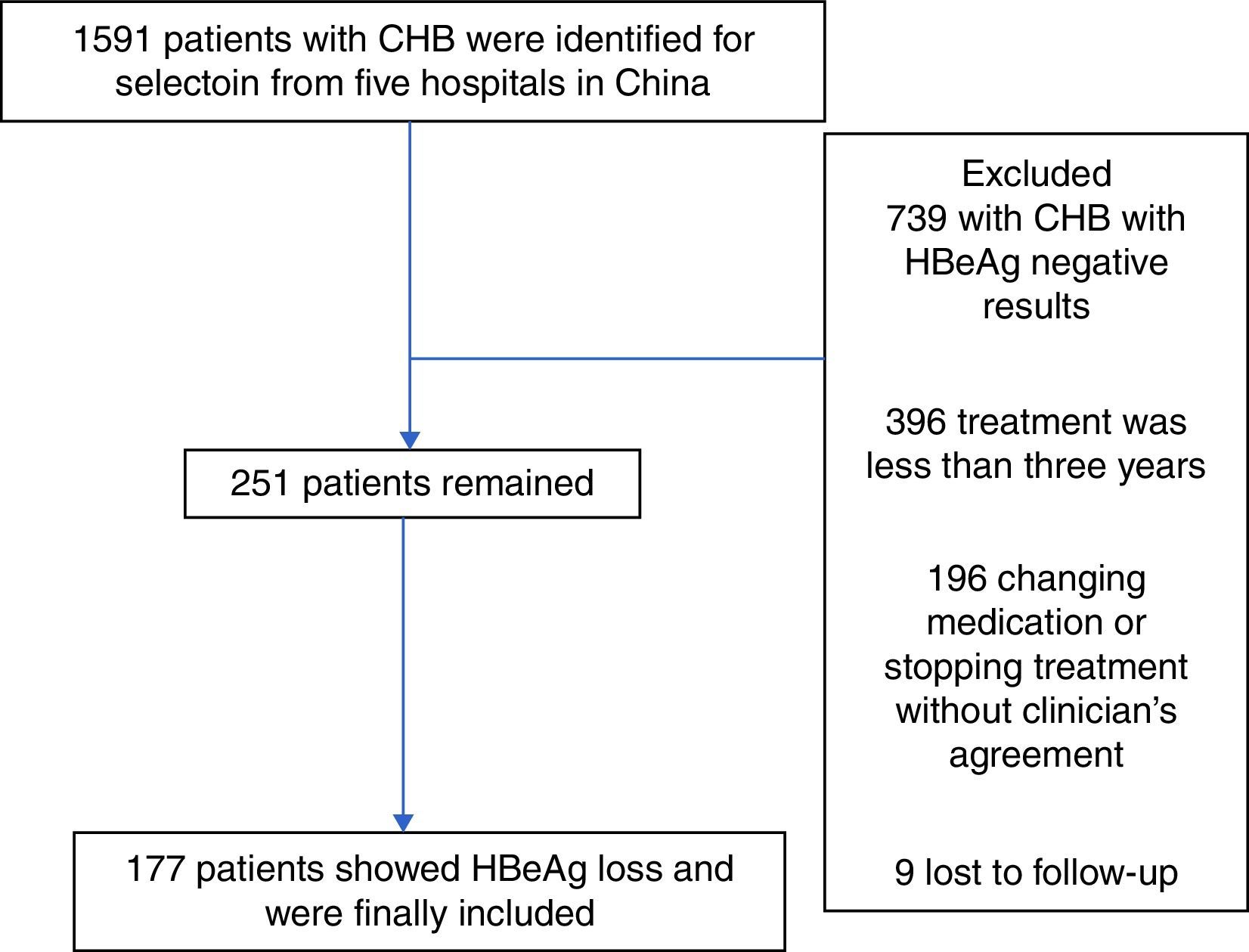
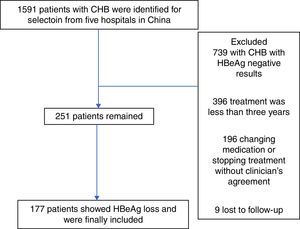
A total of 1591 patients were enrolled. Inclusion criteria were: ≥18 years of age, no previous HBV treatment, and detectable HBeAg and negative anti-HBe at baseline. Patients were excluded if there was any evidence of autoimmune hepatitis, metabolic liver diseases, heavy alcohol abuse, hepatitis C virus, hepatitis D virus, hepatitis E virus, human immunodeficiency virus (HIV), recent exposure to hepatotoxic drugs, and pregnancy for women. Patients with changed anti-HBV agents (switching to an alternate NAs monotherapy, IFN therapy, or adding another anti-HBV medication) were also excluded. A total of 251 patients were finally included in the study but only 177 were fully assessed with recorded HBeAg loss. Patient clinical records, including laboratory results and adherence reports, were reviewed using a self-report questionnaire.
Laboratory examinations were generally conducted at baseline and at 12–24 week intervals. Serum levels of ALT, alpha fetoprotein (AFP), HBV DNA, HBeAg, anti-HBe, HBsAg, and anti-HBs were evaluated. Serum HBV DNA load in CHB patients was detected by real time polymerase chain reaction (PCR) in our Hospital, and detection limits were 500 and 108copies/mL, for lower and upper levels, respectively. HBeAg and HBsAg were measured quantitatively or qualitatively (described as “positive” or “negative”).
The study protocol was approved by the Ethics Committee of our Hospital, and the requirement for written informed consent was waived.
Assessment of treatment outcomesIn this study, HBeAg seroconversion was defined as loss of HBeAg with the development of anti-HBe, accompanied with complete viral suppression (undetectable levels of HBV DNA replication, <500copies/mL) and normalization of ALT (<40IU/L).
Adherence measurementThree questions regarding adherence to anti-HBV NAs were included in a self-administered questionnaire at the last follow-up. The first two were adapted from the questionnaire established for HIV-infected patients by the AIDS Clinical Trial Group.14 The third question corresponded to a visual analogue scale (VAS)15 (Table 1).
Hepatitis B Medication Study.
| Thank you for participating in our Hepatitis B Medication Study. Your responses are very important to us. The information we learn about Hepatitis B medications may help improve the care of other patients who have Hepatitis B. The information you provide on this form will be kept confidential. We will not present this information in any way that would reveal your identity. In addition, we will not share the information provided in this questionnaire with other physicians. |
| 1. How many days have you missed any of your Hepatitis B medicine during the last 30 days? A. None B.–––––––Days 2. Why did you miss your Hepatitis B medicine(s) in the last 30 days? (Can choose more than one option) A. I did not miss any doses in the last 30 days. B. I forgot to take my medicine. C. I do not have the money to pay for my medicine. D. I was too busy doing other things. E. I was travelling or away from home. F. The medicine makes me sick. G. I did not want to take my medicine in the presence of others, which could raise suspicion of me having a disease. H. I did not think I needed it. 3. Visual analogue scale (VAS): put a cross on the line below showing how you consider to have followed the treatment during the last three months: from 0 (=did not followed treatment at all) to 10 (=no omission of antiviral drug dose taking). |
Adherence: 1 or less day of missed medicine in the last 30 days, and VAS=10;
Non-adherence: 2 or more days of missed medicine in the last 30 days, or VAS≤9.
Patients were classified as fully adherent if skipping no more than one medication dose, with a VAS score of 10. Other patients were classified in the non-adherence group. This approach tends to minimize adherence inflation using self-reporting to healthcare providers.16
Statistical analysisDescriptive statistics were reported as number and percentage (%) for categorical variables, and mean±SD or median (range) for continuous variables. Groups of patients with different NAs and HBeAg seroconversion status were compared by χ2-test, log-rank test, Student's t-test, and one-way analysis of variance (ANOVA) for categorical and continuous variables, respectively. Kaplan–Meier analysis was used to assess cumulative incidence rates over time. Cox proportional hazard regression analysis was used to identify independent factors significantly associated with HBeAg seroconversion, with Wald χ2 values and hazard ratios (HR) calculated.
Two-tailed p<0.05 was considered statistically significant. The SPSS software version 17 (SPSS, Chicago, IL, USA) was used for statistical analyses.
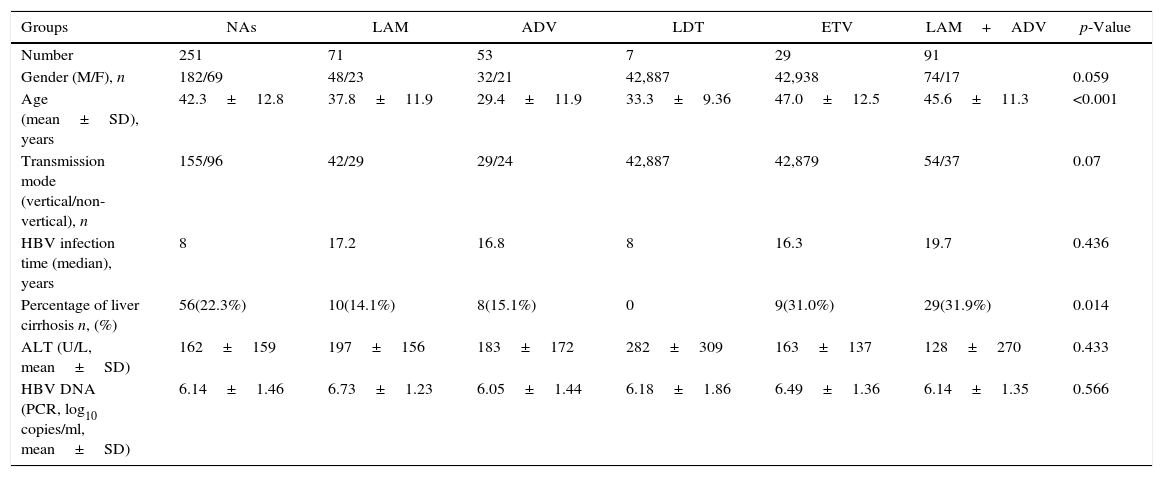
ResultsBaseline characteristics of the included subjectsThe patient flowchart is shown in Fig. 1. Of the 1591 patients, 739 were excluded with chronic HBV infection and HBeAg negative results. A total of 396 additional individuals were excluded for treatment duration below three years, and an additional 196 patients for changing medication/stopping treatment without a clinician's agreement and not conforming to the treatment strategy. Nine patients were lost to follow-up. Finally, 251 eligible patients remained, but only 177 were included and fully studied with recorded HBeAg loss. The number of CHB patients treated with LAM, ADV, LDT, ETV, and LAM/ADV combination were 71, 53, 7, 29, and 91, respectively. Median age was 42.3 years (range, 18–72), and 72.5% of patients were male (182/251). 56 of the 251 patients (22.3%) were diagnosed with liver cirrhosis; 155 patients (61.8%) had vertical transmission modes. Demographic and laboratory characteristics at the time of diagnosis are shown in Table 2.
Baseline characteristics of patients.
| Groups | NAs | LAM | ADV | LDT | ETV | LAM+ADV | p-Value |
|---|---|---|---|---|---|---|---|
| Number | 251 | 71 | 53 | 7 | 29 | 91 | |
| Gender (M/F), n | 182/69 | 48/23 | 32/21 | 42,887 | 42,938 | 74/17 | 0.059 |
| Age (mean±SD), years | 42.3±12.8 | 37.8±11.9 | 29.4±11.9 | 33.3±9.36 | 47.0±12.5 | 45.6±11.3 | <0.001 |
| Transmission mode (vertical/non-vertical), n | 155/96 | 42/29 | 29/24 | 42,887 | 42,879 | 54/37 | 0.07 |
| HBV infection time (median), years | 8 | 17.2 | 16.8 | 8 | 16.3 | 19.7 | 0.436 |
| Percentage of liver cirrhosis n, (%) | 56(22.3%) | 10(14.1%) | 8(15.1%) | 0 | 9(31.0%) | 29(31.9%) | 0.014 |
| ALT (U/L, mean±SD) | 162±159 | 197±156 | 183±172 | 282±309 | 163±137 | 128±270 | 0.433 |
| HBV DNA (PCR, log10 copies/ml, mean±SD) | 6.14±1.46 | 6.73±1.23 | 6.05±1.44 | 6.18±1.86 | 6.49±1.36 | 6.14±1.35 | 0.566 |
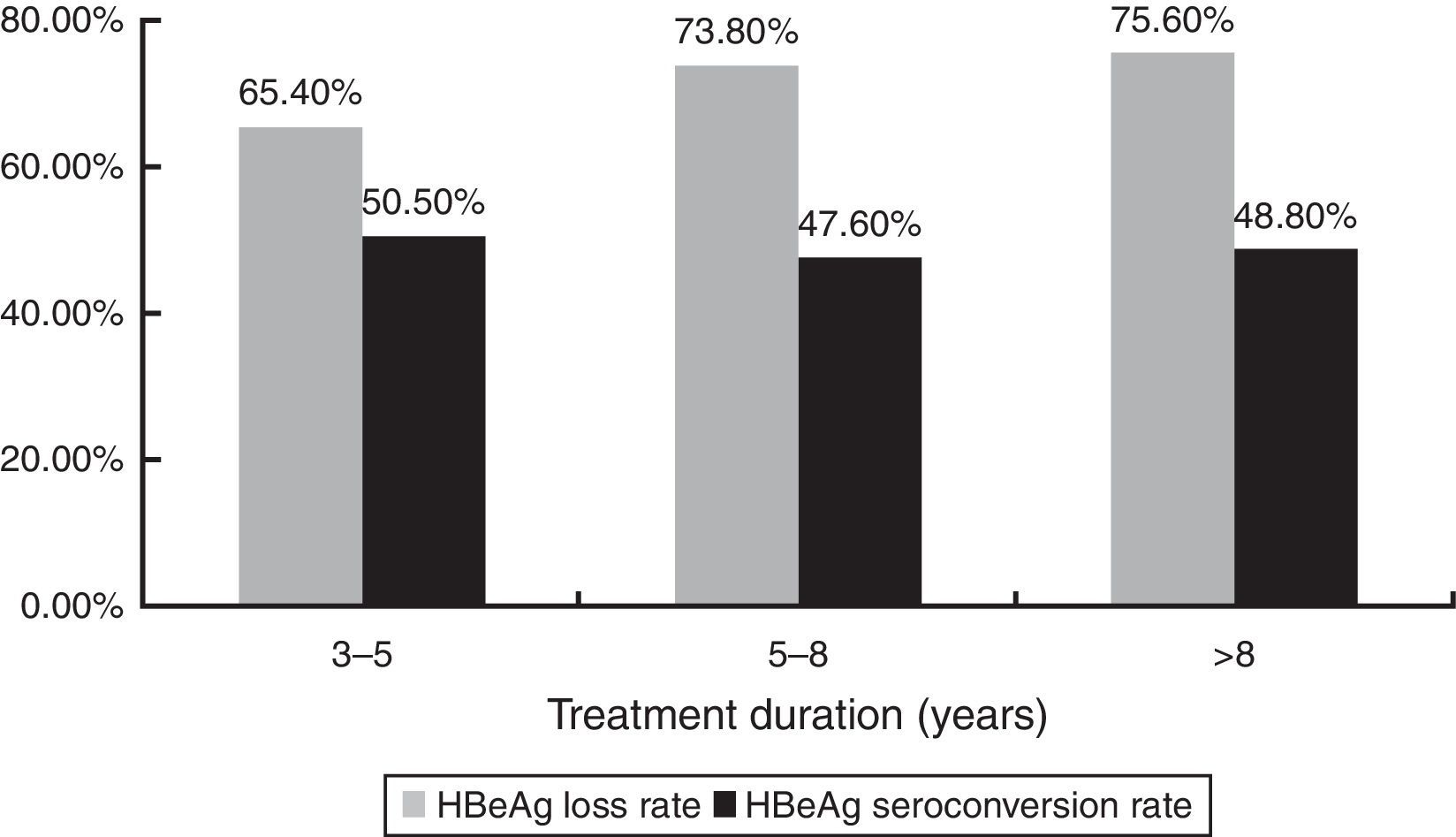
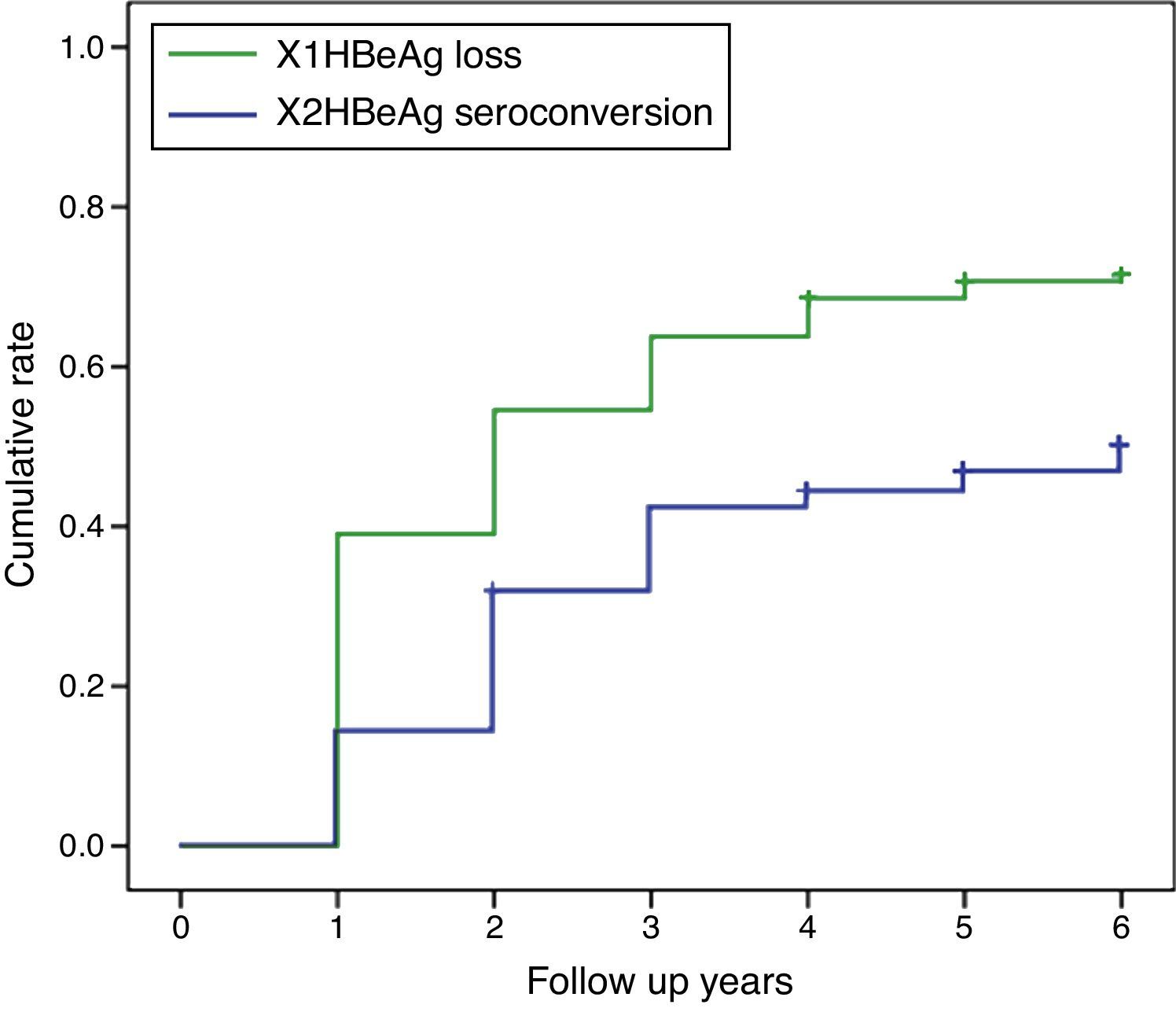
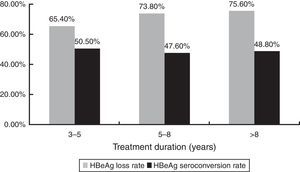
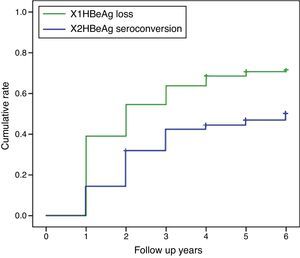
HBeAg loss and seroconversion were observed in 70.5% (177/251) and 49.0% (123/251) of patients, respectively. The total rates of HBeAg loss for patients with treatment duration beyond three, five, and eight years were 65.4% (70/107), 73.8% (76/103), and 75.6% (31/41), respectively; however, total HBeAg seroconversion rates were only 50.5% (54/107), 47.6% (49/103), and 48.8% (20/41), respectively (Fig. 2). The cumulative rates of HBeAg loss and seroconversion are shown in Fig. 3. In real clinical setting, cumulative HBeAg loss rates were 39%, 54.6%, 63.7%, 70.7%, and 74.4% after one, two, three, five, and eight years of treatment, respectively. Meanwhile, cumulative HBeAg seroconversion rates were only 14.3%, 32.7%, 43%, 46.9%, and 50.5% in one, two, three, five, and eight years, respectively.
LAM, ADV, and ETV were well tolerated, and no patients discontinued treatment due to adverse events. No patients died during the study. Creatine kinase elevation (mild) occurred during the first year in four patients on LDT monotherapy.
Adherence to HBV medication is suboptimalA total of 206 patients (82.1%) fully adhered to HBV medication. At the last follow-up, 45 patients reported to have missed HBV medication for ≥1 day in the last 30 days. The most common reasons for missing medication were forgetfulness (n=27) and financial difficulties (n=7).
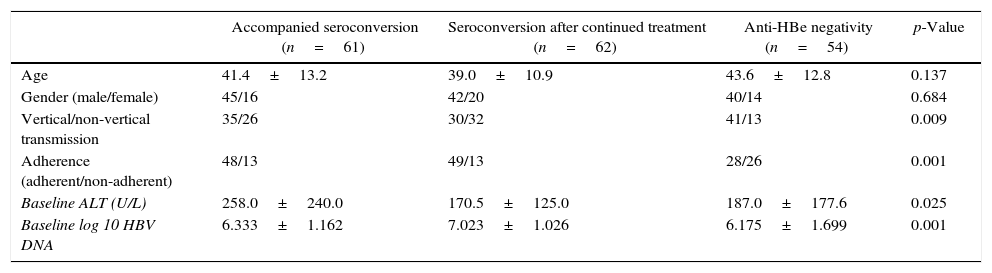
Independent predictors of HBeAg seroconversion in patients with NAs induced HBeAg lossHBeAg loss was observed in 70.5% (177/251) of the patients. In 61 patients, HBeAg loss was accompanied with anti-HBe production; 62 achieved HBeAg seroconversion after a median time on therapy and follow-up of 48 weeks; the remaining 54 patients maintained HBeAg and anti-HBe negative after a median therapy and follow-up of 245 weeks at the end of the study. Univariate analysis revealed that vertical/non-vertical transmission (p=0.009), adherence (p=0.001), baseline ALT (p=0.025), and baseline log HBV DNA (p=0.001) were significantly different among groups as shown in Table 3. These factors were entered to a Cox proportional hazards model, and adherence (Wald χ2=12.228, p<0.001, HR=2.203), high ALT level (Wald χ2=14.750, p<0.001, HR=2.049), and non-vertical transmission (Wald χ2=7.570, p=0.006, HR=1.656) were independent predictive factors of HBeAg seroconversion in patients with NAs induced HBeAg loss (Table 4). The effects of the three identified factors on HBeAg seroconversion were further assessed by the Kaplan–Meier method (Fig. 3).
Univariate analysis of potential predictive factors of HBeAg seroconversion.
| Accompanied seroconversion (n=61) | Seroconversion after continued treatment (n=62) | Anti-HBe negativity (n=54) | p-Value | |
|---|---|---|---|---|
| Age | 41.4±13.2 | 39.0±10.9 | 43.6±12.8 | 0.137 |
| Gender (male/female) | 45/16 | 42/20 | 40/14 | 0.684 |
| Vertical/non-vertical transmission | 35/26 | 30/32 | 41/13 | 0.009 |
| Adherence (adherent/non-adherent) | 48/13 | 49/13 | 28/26 | 0.001 |
| Baseline ALT (U/L) | 258.0±240.0 | 170.5±125.0 | 187.0±177.6 | 0.025 |
| Baseline log 10 HBV DNA | 6.333±1.162 | 7.023±1.026 | 6.175±1.699 | 0.001 |
The aim of this study was to evaluate the HBeAg seroconversion rate in patients with CHB treated in real clinical setting, and to determine factors that predict seroconversion with long term NAs treatment. The results showed that cumulative HBeAg seroconversion rates during NAs therapy were 14.3%, 32.7%, 43.0%, 46.9%, and 50.5% at one, two, three, five, and eight years, respectively. Adherence to HBV medication, high ALT level, and non-vertical transmission were predictive factors of HBeAg seroconversion.
In registered clinical trials as low as 20% of CHB patients treated with NAs achieve HBeAg seroconversion after one year of treatment, despite small increases in LDT-treated patients.10,17–19 However, registered clinical trials strictly select patients on the basis of inclusion criteria such as age, gender, and baseline ALT levels, all of which are predictive factors of anti-HBV responses. Clinical trials also require close adherence to strict protocol guidelines, which may improve the measured medication adherence levels and consequently the HBeAg seroconversion rates. Thus, the HBeAg seroconversion rate might be even lower in real clinical setting. Indeed, in a recent study conducted in real clinical setting in the United States, the HBeAg seroconversion rate at month 12 was as low as 8.2%.9 Another study reported an even lower rate of 4.8% at 12 months of ETV treatment.8 In this study the HBeAg seroconversion rate at one year was 14.3%. Potential reasons for the difference between HBeAg seroconversion rates in the current study and the previous trials may include differences in baseline characteristics, adherence of the patient population, and the genotypic distribution of HBV. In addition, this study retrospectively recruited patients on the basis of NAs treatment duration. Another explanation for the seemingly higher HBeAg seroconversion rate in the current study may be selection bias because we included only patients still being treated, and some patients might have been excluded from follow-up due to suboptimal anti-HBV responses or drug resistant mutations during the first three years.
In clinical trials, however, after long-term follow-up, the HBeAg seroconversion rate increases with therapy duration to more than 30% and 40% at three and five years, respectively.7,20–24 The current study found a similar HBeAg seroconversion rate of 43% and 46.9% at three and five years, respectively, corroborating previous studies in real clinical setting.8,9 Thus, medical adherence might only affect anti-HBV responses during the initial year. Several studies have also reported that persistence and adherence rates to anti-HBV agents and other medicines for chronic diseases dramatically drop after the first six months of therapy.25–28 Although an even longer duration of eight years on NAs treatment was associated with increased HBeAg seroconversion rate of more than 50%, the NAs induced HBeAg seroconversion is not durable.29 Thus, in real-life clinical settings, HBeAg-positive patients should be counselled about the possibility of long treatment duration being required to achieve and maintain HBeAg seroconversion.
Increases in HBeAg loss and seroconversion rates were not parallel because the rate of HBeAg loss continued to increase with time while HBeAg seroconversion almost levelled off after three years. This may be partially related to insufficient anti-HBV related immune responses in some patients with NAs induced HBeAg loss.
Prediction of the response to anti-HBV treatment has become increasingly important in therapy individualization for CHB patients. Baseline predictive factors can assist physicians to select the best timing for treatment initiation as well as antiviral agents. Baseline ALT and HBV DNA levels are important factors for HBeAg seroconversion. Indeed, patients with higher baseline ALT levels and lower HBV DNA replication have a higher probability of HBeAg seroconversion, both in the natural course30,31 and during NAs or interferon therapy.7,10,32,33 This may be related to more severe inflammatory processes, and subsequent more rapid viral loads and HBeAg titre decrease in patients with higher baseline ALT levels.
Adherence was found to be an important predictor of HBeAg loss/seroconversion in the current study. Its predictive value of virological suppression or breakthrough in patients receiving anti-HBV NAs treatment was also demonstrated in clinical practice.16,34 However, this is the first study of this kind in the Chinese population in real clinical setting. Thus, adherence might be seriously taken into consideration while treating CHB patients with NAs, especially during the first year. Indeed, more time should be spent counselling patients regarding the importance of adherence to HBV treatment and the risks of medication discontinuation.
The strengths of the current study are the relatively large sample size, availability of data for the four different NAs used in routine clinical practice, and a broad representation of the database. However, several limitations of the study should be mentioned. First, selection bias was inevitable in this retrospective analysis. For instance, although HBeAg seroconversion depends on HBV genotype, we did not determine HBV genotypes, which could not be assessed as an influencing factor in this study. In addition, treatment duration may result in an overestimation of anti-HBV responses. Second, patients who switched to or added other anti-HBV medications due to suboptimal responses or drug resistant mutations were excluded. This would definitely impact HBeAg seroconversion rates, although the effect is expected to be relatively small. Third, the number of LDT-treated patients in the current data set was small, which limited our ability to make recommendations regarding the indicated agent. Fourth, adherence was evaluated only once, at last follow-up, and related data during the first three years were missing. However, adherence to antiviral therapy might be lower than expected, leaving patients at risk of delayed HBeAg seroconversion, increased virological breakthrough, and disease progression.
In real clinical setting, cumulative HBeAg seroconversion rates during NAs therapy are lower in the first year but similar after long term treatment to values reported in clinical trials. Adherence to HBV medication, higher ALT level, and non-vertical transmission are predictive factors of HBeAg seroconversion in patients treated with NAs.
Conflicts of interestThe authors declare no conflicts of interest.