HAM/TSP (HTLV-1-associated myelopathy/tropical spastic paraparesis) is a slowly progressive disease, characterized by a chronic spastic paraparesis. It is not known if the disease carries an independent risk for cardiovascular disease. The objective of this study was to evaluate the cardiovascular risk profile related to HAM/TSP and compare it with the general population.
MethodsThis was a cross-sectional study, with a control group. HAM/TSP patients were evaluated using cardiovascular risk scores (ASCVD RISK, SCORE and Framingham) and inflammatory markers (ultrasensitive CRP and IL-6), and compared with a control group of healthy individuals. We also evaluated the correlation between cardiovascular risk and the functional status of patients with HAM/TSP evaluated by the FIM scale.
ResultsEighty percent of patients in this study were females, mean age of 51 years (11.3). The control group showed an increased cardiovascular event risk in 10 years when ASCVD was analyzed (cardiovascular risk ≥7.5% in 10 years seen in 43% of patients in the control group vs. 23% of patients with HAM/TSP; p=0.037). There was no difference in ultrasensitive CRP or IL-6 values between the groups, even when groups were stratified into low and high risk. There was no correlation between the functional status of HAM/TSP patients and the cardiovascular risk.
ConclusionsIn this study, the cardiovascular risk profile of patients with HAM/TSP was better than the risk of the control group.
Human T-lymphotropic virus type 1 (HTLV-1) was the first known human retrovirus, described in 1979 by Poiesz.1 It is estimated that 15–20 million people are affected worldwide.2 The incidence of HTLV-1 infection is higher in Japan, Africa, and Caribbean Islands, where it may affect 5% or more of the population.3 The virus was initially associated with adult T-cell leukemia; subsequently, myelopathy (HTLV-1-associated myelopathy/tropical spastic paraparesis – HAM/TSP), uveitis, and infective dermatitis were also associated with the virus.4–7 Only 5–10% of patients, however, develop clinical manifestations.
HAM/TSP is a slowly progressive disease. It is characterized by a chronic spastic paraparesis, neurogenic bladder, neurogenic bowel, spasticity and neuropathic pain. Because these symptoms are similar to those found in patients with other spinal cord diseases (traumatic or non-traumatic), their treatment overlaps, consisting mostly of supportive measures and rehabilitation. There is currently no antiviral treatment for the disease. Once HAM/TSP develops, it is usually progressive and irreversible.
In the past, pulmonary and renal complications were the main causes of morbidity and mortality of patients with traumatic spinal cord injury.8,9 However, cardiovascular disease is now one of the leading causes of death.8,10,11 Risk factors such as diabetes, low HDL-cholesterol (HDL-C), sedentary life-style, and smoking have a higher prevalence among patients with spinal cord injury.8,10,12–14
Few studies describe the cardiovascular involvement related to HTLV-1. In 2014, Layegh et al.,15 in a cross-sectional study assessed atherosclerosis in patients with HTLV-1. The authors found that HTLV -1 infected patients had greater carotid intima-media thickness than age-mached healthy controls. In 1996, Stuver et al. found increased incidence of cardiovascular diseases and EKG changes in patients infected with HTLV-1.16 In 2013, Shabestari et al. found increased prevalence of HTLV-1 among patients undergoing angiography.17
The Human Immunodeficiency Virus (HIV), another retrovirus, is associated to increased cardiovascular risk. Freinberg et al. studied 82,459 patients with HIV during 5.9 years. After adjusting for comorbidities and cardiovascular risk scores, they found that HIV was an independent cardiovascular risk factor, raising the risk of a myocardial infarction by 50%. The mechanism by which HIV increases the risk of myocardial infarction is not known; it is thought that viral induced inflammation and endothelial dysfunction may play a role.18 Anti-retroviral therapies, especially protease inhibitors are also related to higher cardiovascular risk.19 As HIV and HTLV-1 are both retrovirus and share similar replication enzymes such as retroviral proteases,20 one could hypothesize that HTLV-1 infection could also be an independent cardiovascular risk factor.
The objective of the present study was to evaluate the cardiovascular risk profile of patients with HAM/TSP and compare it with the general population.
MethodsStudy designThis was a cross-sectional study, with a control group.
Patients and settingsThe study group was comprised of HAM/TSP patients followed in a rehabilitation Hospital in the city of Salvador, Brazil from July 2014 to October 2015. All patients had the diagnosis of HAM/TSP defined according to the Castro–Costa criteria.21 Patients with other possible causes for spinal cord lesions were excluded. Patients with possible causes for changes in inflammatory tests such as acute infections, rheumatologic diseases, inflammatory bowel disease, and use of corticosteroids or nonsteroidal anti-inflammatory drugs were also excluded.
Mid level employees of Hospital Sarah Salvador constituted the control group. They were paired in a 1:1 proportion, matching for sex and age ±5 years.
Socio-demographic data, physical examination and laboratory testsData were collected during individualized interviews, using a standardized questionnaire. Socio-demographic data and information about HAM/TSP disease (such as duration of the disease and comorbidities associated to the myelopathy) were collected.
Weight was obtained in a calibrated scale with patients using light clothing. For walking patients and in the control group, height was obtained using the metric measurement bar attached to an anthropometric scale. In wheelchair bound patients, height was obtained with the patient in the supine position, using a flexible inelastic tape, measuring segments of the body from the heel to head. Body mass index (BMI) was calculated by the Quetelet formula (weight/height2).
An inelastic flexible tape was used to measure waist circumference. The measurement was obtained with the patient standing upright, with arms outstretched, after a normal expiration, at the midpoint between the last rib and the anterior superior iliac crest.22 Wheelchair bound patients were assisted to a standing position with the use of a walker, parallel bars or a standing table for this measurement, whenever possible. The waist circumference was not measured if a standing position was not attainable. Two measurements were performed, and the average of the values was recorded.
Blood pressure was measured with aneroid sphygmomanometer with patients seated with supported upper extremity at the heart level. Patients were at rest for at least 10min and had not smoked or drank coffee 30min prior to the measurement.23 Patients with neurogenic bladder underwent bladder catheterization before blood pressure measurement. Two measurements were performed, the second one minute after the first, and the average of the values was recorded.23
Presence of metabolic syndrome was assessed by using the International Diabetes Federation criteria as previously described.24 Metabolic syndrome refers to a cluster of associated symptoms composed of impaired fasting glucose, abdominal obesity, hypertension, and dyslipidemia. It is associated with an increased risk of cardiovascular morbidity and mortality. The increased amount of visceral fat together with a chronic inflammatory state predisposes to the development of arteriosclerosis.
In the HAM/TSP group, the functional status was evaluated using the Functional Independence Measure (FIM) scale, a highly accepted and utilized tool for neurological patients that has been validated in Brazil.25 It is used primarily in patients with neurological sequelae and evaluates 18 categories that are scored from 1 to 7 determinants of the level of dependency to perform a task. The sum of the points obtained will classify the patient into four subscores: complete dependency, modified dependency (assistance of up to 50% of the task), modified independence (assistance of up to 25% of the task), and total independence.
Blood samples collected after a 12-hour fasting included glucose, total cholesterol, LDL cholesterol (LDL-C), HDL cholesterol (HDL-C), triglycerides (spectrophotometry), complete blood count (Flow Cytometry, impedance and optical microscopy), creatinine (Jaffé), ultrasensitive C reactive protein (CRP) (nephelometry, BN Prospec®), interleukin-6 (IL-6) (chemiluminescence, Simens Imullite thousand®), proviral load (Polymerase Chain Reaction) for the HAM/TSP patients and HTLV-1 test detection (ELISA) for the control group. All tests were performed in hospital's laboratories at the Sarah Network.
Cardiovascular risk evaluationThree different cardiovascular risk scores were used: Atherosclerotic Cardiovascular Disease Risk (ASCVD Risk), the Framingham score, and the European Systematic Coronary Risk Evaluation (SCORE). We compared the absolute value of 10-year cardiovascular risk of each score. The main outcome of this study, however, was the cardiovascular risk assessed by ASCVD RISK. The ASCVD RISK was chosen since it is a score developed from a large number of patients including patients with different racial background, and it predicts the risk for fatal and non-fatal events. Patients were dichotomized into two groups: low risk/no need for therapeutic intervention, and intermediate and high risk/need for therapeutic intervention.
The ASCVD RISK was created in 2013 in a partnership between the American Heart Association, American College of Cardiology and the National Heart, Lung and Blood Institute. It uses data from Cohorts such as the ARIC study (Atherosclerosis Risk in Communities), CARDIA study (Coronary Artery Risk Development in Young Adults) and the Framingham study. These patients were followed for at least 12 years, and were mainly white non-Hispanics and African Americans. The score was created to calculate the cardiovascular risk of adults between 40 and 79 years of age. It estimates the risk of developing fatal or non-fatal acute myocardial infarction or stroke in 10 years based on the variables gender, age, race, total cholesterol and HDL-C, systolic blood pressure, smoking, and diabetes.26 Patients with diabetes, symptomatic cardiovascular disease, or a cardiovascular risk ≥7.5% are considered high risk patients.
The Framingham Risk Score was created from data obtained in the Framingham Heart Study. This cohort study started in 1948 in the city of Framingham, Massachusetts, with about 5000 participants.27 In 2008 a score for assessment of cardiovascular risk in primary care was established by estimating the probability of myocardial infarction, stroke, peripheral arterial disease, and heart failure in the next 10 years. This score uses as variables gender, age, smoking, diabetes, systolic blood pressure, use of medications for high blood pressure, total cholesterol, and HDL-C.28 The Framingham Risk Score classifies patients into three groups: low risk (<5% risk of cardiovascular event in 10 years), intermediate risk (5–20% for men and 5–10% for women), and high risk (>20% for men and >10% for women).
The European SCORE is a tool created in 2003 with data from 12 European cohorts, including 205,178 patients followed between 1970 and 1988. It estimates the risk of fatal cardiovascular events in 10 years, such as myocardial infarction, stroke, and aortic aneurysm based on the variables gender, age, total cholesterol, systolic blood pressure, and smoking status.29 The European SCORE also classifies patients into three groups: low risk (<1% risk of cardiovascular event in 10 years), intermediate risk (1–5%), and high risk (>5%). Patients with diabetes are considered high risk.
The cardiovascular risk was also evaluated using ultrasensitive CRP and IL-6, inflammatory markers that are elevated in patients with increased cardiovascular risk.30,31 Patients were considered to have a high cardiovascular risk if IL-6>3.4pg/ml and/or CRP>2mg/l.
Statistical analysisAll analyses were performed by the statistical package SPSS (Statistical Package for Social Sciences) version 21.0.
Categorical variables were compared using a chi-square test with Yates's correction for continuity, as appropriate. Continuous variables were assessed for significant departures from normality. Normally distributed variables were summarized using mean and standard deviation and compared using a t test. Skewed variables were summarized using median and interquartile range (IQR) and compared using a Mann–Whitney test.
We conducted a multivariate analysis by binary logistic regression. We included in the multivariate model factors with p<0.20 in univariate analysis, which had biological plausibility and were not used in the risk score. The final outcome was dichotomous (low cardiovascular risk profile RCV/high cardiovascular risk profile), and the final model obtained by the Backward method.
In HAM/TSP group, the correlation between FIM scale values and cardiovascular risk was evaluated using Spearman's rho.
p-Values were considered significant if less than 0.05.
Sample size calculationThe sample size was calculated to confer statistical power to the preset analysis. Fifty-six patients were needed in each group to provide an 80% power to detect a difference of 28% of patients with intermediate/high cardiovascular risk between the groups. Using data from previous studies10,14,32 we estimated that 65% of the patients with HAM/TSP would have an intermediate/high cardiovascular risk, while the prevalence in the comparative group would be 37%.
Ethical aspectsThe study was in compliance with the guidelines on human research of the Helsinki Declaration and the National Health Council Resolution 466/2012. The study was submitted to the Ethics Committee of the Sarah Network and approved in June, 2014 (CAAE 31338914.2.0000.0022). All participants received detailed information in writing and verbally about the study objectives, risks and benefits involved in the procedures, and signed informed consent before being submitted to the procedures described in methods.
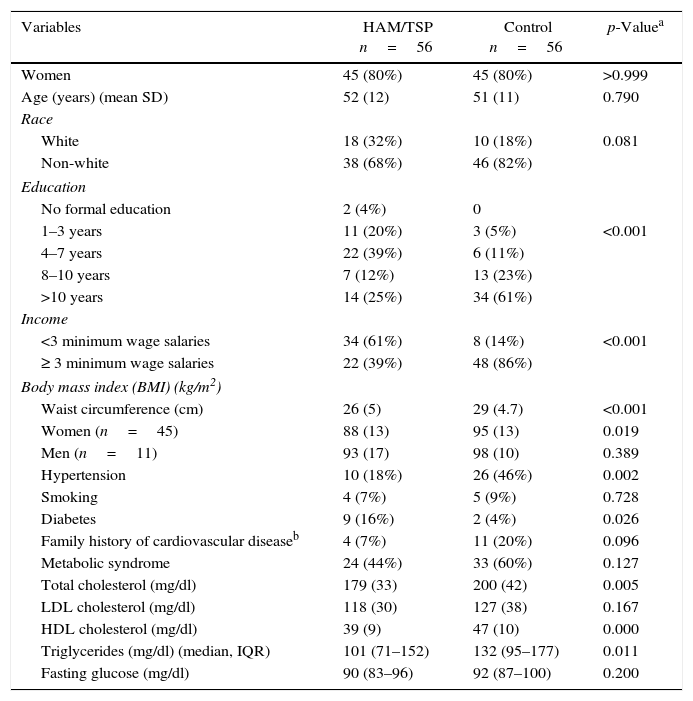
ResultsThe social and clinical characteristics of the patients are presented in Table 1. In both groups, 80% of patients were female with a mean age of approximately 51 years (11.3). There was no difference between groups with respect to race (p=0.081). The groups differed in the educational status (p<0.001) and family income (p<0.001), with the control group presenting with more years of formal education and higher income.
Characteristics of the study samples.
| Variables | HAM/TSP n=56 | Control n=56 | p-Valuea |
|---|---|---|---|
| Women | 45 (80%) | 45 (80%) | >0.999 |
| Age (years) (mean SD) | 52 (12) | 51 (11) | 0.790 |
| Race | |||
| White | 18 (32%) | 10 (18%) | 0.081 |
| Non-white | 38 (68%) | 46 (82%) | |
| Education | |||
| No formal education | 2 (4%) | 0 | |
| 1–3 years | 11 (20%) | 3 (5%) | <0.001 |
| 4–7 years | 22 (39%) | 6 (11%) | |
| 8–10 years | 7 (12%) | 13 (23%) | |
| >10 years | 14 (25%) | 34 (61%) | |
| Income | |||
| <3 minimum wage salaries | 34 (61%) | 8 (14%) | <0.001 |
| ≥ 3 minimum wage salaries | 22 (39%) | 48 (86%) | |
| Body mass index (BMI) (kg/m2) | |||
| Waist circumference (cm) | 26 (5) | 29 (4.7) | <0.001 |
| Women (n=45) | 88 (13) | 95 (13) | 0.019 |
| Men (n=11) | 93 (17) | 98 (10) | 0.389 |
| Hypertension | 10 (18%) | 26 (46%) | 0.002 |
| Smoking | 4 (7%) | 5 (9%) | 0.728 |
| Diabetes | 9 (16%) | 2 (4%) | 0.026 |
| Family history of cardiovascular diseaseb | 4 (7%) | 11 (20%) | 0.096 |
| Metabolic syndrome | 24 (44%) | 33 (60%) | 0.127 |
| Total cholesterol (mg/dl) | 179 (33) | 200 (42) | 0.005 |
| LDL cholesterol (mg/dl) | 118 (30) | 127 (38) | 0.167 |
| HDL cholesterol (mg/dl) | 39 (9) | 47 (10) | 0.000 |
| Triglycerides (mg/dl) (median, IQR) | 101 (71–152) | 132 (95–177) | 0.011 |
| Fasting glucose (mg/dl) | 90 (83–96) | 92 (87–100) | 0.200 |
The control group had a higher prevalence of hypertension (46% versus 18% of patients in HAM/TSP group, p=0.002). The waist circumference of women of this group was also significantly higher (95cm (13) versus 88cm (13) of HAM/TSP group, p=0.019). The incidence of diabetes mellitus was higher in the HAM/TSP group (16% versus 4% of control group, p=0.026); however, the median fasting blood glucose was similar between the groups (90mg/dl in the HAM/TSP group versus 92mg/dl in the control group, p=0.239). The control group had significantly higher total cholesterol level (200mg/dl (42) versus 179mg/dl (33), p=0.007), and triglyceride levels (median of 134mg/dl versus 102mg/dl in HAM/TSP group, p=0.012, Mann–Whitney). HDL-C level was also higher in HAM/TSP group (46mg/dl (10) versus 39mg/dl (9) p<0.001).
The control group had higher BMI (29kg/m2 (4.7) versus 26kg/m2 (5), p<0.001). The prevalence of obese patients (BMI>30kg/m2) was significantly higher in the control group than in the HAM/TSP group (43% versus 16%, p=0.001). There was no significant difference in the prevalence of metabolic syndrome, although we observed a trend toward higher prevalence in the control group (60% versus 44% of HAM/TSP group, p=0.127).
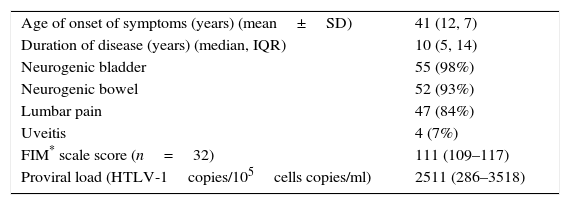
In the HAM/TSP group, the mean age of onset of myelopathy symptoms was 41 years (12.7). The median FIM scale score was 111 (IQR 109–117). The median provirus load was 2510 HTLV-1copies/105 cells copies/ml (IQR 286–3518). Table 2 shows the characteristics of these patients.
HAM/TSP patients characteristics (n=56).
| Age of onset of symptoms (years) (mean±SD) | 41 (12, 7) |
| Duration of disease (years) (median, IQR) | 10 (5, 14) |
| Neurogenic bladder | 55 (98%) |
| Neurogenic bowel | 52 (93%) |
| Lumbar pain | 47 (84%) |
| Uveitis | 4 (7%) |
| FIM* scale score (n=32) | 111 (109–117) |
| Proviral load (HTLV-1copies/105cells copies/ml) | 2511 (286–3518) |
FIM, functional independence measure.
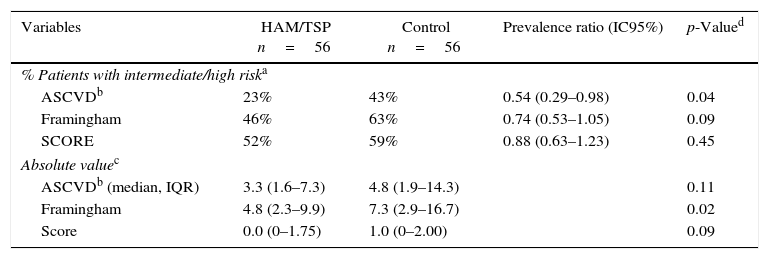
The ASCVD, Framinghan, and SCORE risk scores were calculated for each patient in the HAM/TSP and control groups (Table 3). Both groups had similar ASCVD and SCORE risk scores; however, the control group showed a statistically significant higher Framingham risk score than the HAM/TSP group (median of 7.3% risk of cardiovascular event in 10 years in the control group versus 4.8% in the HAM/TSP group, p=0.024).
Cardiovascular risk scores in HAM/TSP patients and control group.
| Variables | HAM/TSP n=56 | Control n=56 | Prevalence ratio (IC95%) | p-Valued |
|---|---|---|---|---|
| % Patients with intermediate/high riska | ||||
| ASCVDb | 23% | 43% | 0.54 (0.29–0.98) | 0.04 |
| Framingham | 46% | 63% | 0.74 (0.53–1.05) | 0.09 |
| SCORE | 52% | 59% | 0.88 (0.63–1.23) | 0.45 |
| Absolute valuec | ||||
| ASCVDb (median, IQR) | 3.3 (1.6–7.3) | 4.8 (1.9–14.3) | 0.11 | |
| Framingham | 4.8 (2.3–9.9) | 7.3 (2.9–16.7) | 0.02 | |
| Score | 0.0 (0–1.75) | 1.0 (0–2.00) | 0.09 | |
Using the ASCVD RISK score, more patients in the control group were found to have an intermediate/high cardiovascular risk than in the HAM/TSP group (43% of patients in the control group versus 23% of patients with HAM/TSP, p=0.037). Similarly, when using Framingham and SCORE, more patients in the control group were classified as intermediate/high risk than in the HAM/TSP group, although this difference was not statistically significant (Table 3).
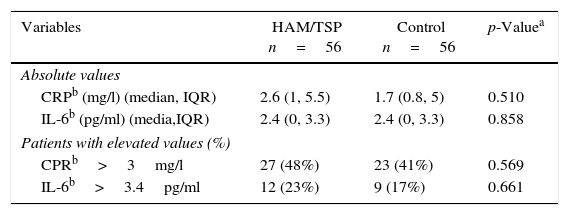
There was no difference between ultrasensitive CRP and IL-6 in both groups when we used either the absolute value or when the groups were categorized into low and high risk patients according to their lab values (Table 4).
Inflammatory markers in HAM/TSP patients and control group.
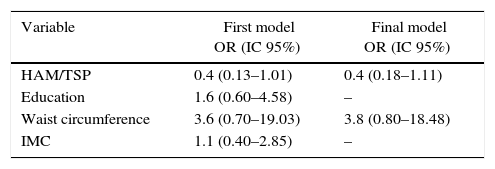
In multivariate analysis, none of the evaluated factors were associated with a better or worse cardiovascular risk profile. Race and income were not included in the model, since race is a variable used in ASCVD RISK. Income showed a strong relationship with education, which could lead to an interaction in the model (Table 5).
Multivariate analysis for the association between education, HAM/TSP, abdominal circumference and BMI, and cardiovascular risk assessed by ASCVD RISK (n=92).
| Variable | First model OR (IC 95%) | Final model OR (IC 95%) |
|---|---|---|
| HAM/TSP | 0.4 (0.13–1.01) | 0.4 (0.18–1.11) |
| Education | 1.6 (0.60–4.58) | – |
| Waist circumference | 3.6 (0.70–19.03) | 3.8 (0.80–18.48) |
| IMC | 1.1 (0.40–2.85) | – |
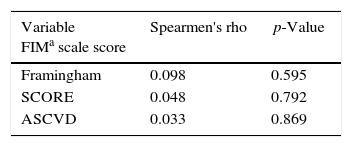
Of the 56 patients in this group, 32 were evaluated by the FIM scale. There was no correlation between the FIM scale and the cardiovascular risk of these patients (Table 6).
Correlation between cardiovascular risk score and FIM scale score.
| Variable FIMa scale score | Spearmen's rho | p-Value |
|---|---|---|
| Framingham | 0.098 | 0.595 |
| SCORE | 0.048 | 0.792 |
| ASCVD | 0.033 | 0.869 |
To our knowledge, this is the first study evaluating the cardiovascular risk profile of patients with HAM/TSP. Although we initially postulated that patients with HAM/TSP could have a worse cardiovascular risk profile than the normal population, this was not seen in our study. In fact, more patients in the control group had higher cardiovascular scores as measured by ASCVD. The median score measured by Framinghan cardiovascular risk score was also higher in this group.
This result can be partially explained by the fact that the prevalence of high blood pressure, total cholesterol, triglycerides, and waist circumference was significantly higher in the control group. However, it is important to note that HAM/TSP patients presented some characteristics that may actually lower their cardiovascular risk, such as low total cholesterol and low blood pressure. Although spinal cord injury patients usually have high prevalence of dyslipidemia, this may not be true for HAM/TSP patients. Doria et al.,33 evaluated 54 HTLV-1 patients for atherosclerosis using carotid artery Doppler. The authors found that only 35% of the patients had atherosclerosis and attributed this fact to the low dyslipidemia prevalence among the studied patients. Furthermore, blood pressure is also an important risk factor for cardiovascular disease and for this reason it is considered in all scores that assess cardiovascular risk. Changes in autonomic control mechanisms were also described in these patients with dysfunction in the sympathetic nervous system. Ohishi et al.,34 showed that HTLV -1 patients had lower average blood pressure in 24h and higher average sleep heart rate when compared to a control group. This study took place in the Japanese city of Nagasaki, and compared 23 HTLV patients with 23 age and sex matched healthy controls. As blood pressure and cholesterol are important cardiovascular risk factors, this can mean that HAM/TSP patients may actually have, in fact, a better cardiovascular risk profile than apparently healthy individuals.35,36
There are other possible explanations for our results, including the application of the cardiovascular scores in the HAM/TSP population. Finnie et al.,10 evaluated the cardiovascular risk of 75 patients with spinal cord injury. Nineteen percent of the patients had an intermediate/high cardiovascular risk when Framingham score was used, against 37% when they evaluated CRP. The authors argued that the Framingham risk score may underestimate the cardiovascular risk of spinal cord injury patients, because it may not consider some specific characteristics of this population. This finding may also be true for the other two scores used in this study.
Another factor that may have played a role in our findings is that the patients in our HAM/TSP group are closely monitored in a specialized rehabilitation center and receive high level medical care. They are evaluated at least annually or semi-annually by a specialized, multidisciplinary medical team, with emphasis not only in rehabilitation, but also in preventive medicine. The fact that these patients had a higher prevalence of history of diabetes than the control group, but their fasting blood glucose levels were similar, demonstrates the close monitoring and control of comorbidities in this group. Considering that they are so strictly monitored, their risk factors may be minimized.
The control group was found to have a higher level of education and family income, which is usually associated with lower cardiovascular risk.37–39 Although one may postulate that if the groups were similar in their social economic status, the difference between the cardiovascular risk could had been even greater (with HAM/TSP patients showing with a more significant lower cardiovascular risk), in our multivariate analysis, education level was not associated with cardiovascular risk.
Despite the fact that HAM/TSP is considered a chronic inflammatory disease, we did not find differences when evaluating ultrasensitive CRP and IL-6 between the groups. About 45% of patients in this study had elevated levels of ultrasensitive CRP, and about 20% had elevated levels of IL-6, with no difference between the HAM/TSP and the control group. Elevated levels of CRP and IL-6 were described by previous studies in HTLV-1 patients.40,41 One possible explanation for our finding is the higher prevalence in the control group of factors also associated to elevated levels of inflammatory markers, such as obesity and hypertension.42,43
The functional capacity of patients with HAM/TSP in this study varied widely, with the score by FIM scale ranging from 72 to 121. A worse functional performance could be related to a higher cardiovascular risk using the hypothesis that most compromised patients are more sedentary.44 However, this study did not demonstrate a positive correlation between cardiovascular risk and the result of the FIM scale.
Our study had limitations. It was a cross sectional study and the number of patients was small. It was designed to detect a difference of 28% prevalence of intermediate/high cardiovascular risk among patients with HAM/TSP and the control group. If the difference between the two groups was smaller than this, we would need a bigger sample size to detect this difference. Although our analysis did not find an important impact of the socio-demographic characteristics on the results, similar baseline characteristics would provide more reliable results. Since we did not evaluate asymptomatic HTLV-1 patients, we can not tell if the results found in the study were due to the myelopathy or to the virus itself.
ConclusionIn this study, the cardiovascular risk profile of patients with HAM/TSP followed in a rehabilitation hospital in Salvador, Brazil was better than the risk profile of the control group of healthy individuals. Further studies are needed to better clarify this issue.
FundingSARAH Network of Rehabilitation Hospitals. The funding source had no involvement during the research and preparation of the article.
Conflicts of interestThe authors declare no conflicts of interest.
We thank Gabriela Modesto for helping with the FIM score collecting data and Alfredo Silva for helping with the statistic review. Supported by SARAH Network of Rehabilitation Hospitals,
This article is part of Fábio Prado's MSc Thesis for the Bahiana School of Medicine and Public Health Post Graduate Course.
This article is part of Fabio Prado MSc Thesis of Bahiana School of Medicine and Public Health Post Graduate Course.