Evidence-based strategies to improve the hepatitis B virus (HBV) vaccination coverage rates might help to reduce the burden caused by co-infection with HBV and human immunodeficiency virus (HIV). In this study, the aim was to evaluate the vaccination coverage and immunity against HBV among HIV-infected individuals in South Brazil, and identify factors that are associated with compliance patterns and antibody reactivity. Three hundred HIV-infected men and women were included in this survey. The patients answered a standardized questionnaire, and vaccination cards were checked in order to assess hepatitis B vaccine status. A blood sample was collected for quantitative determination of antibody to hepatitis B virus surface antigen (anti-HBs). Participants were also evaluated for their CD4 cell count and HIV viral load. The overall vaccination coverage of HBV vaccination found in this study (57.4%) was lower than that was previously reported in South Brazil. Anti-HBs levels >10IU/L were observed in 47.0% of the studied population. A significant inequality in the coverage rates and antibody reactivity was found in favor of patients with better economic status. In conclusion, the results indicate the need for improvement in the HBV vaccination coverage among HIV carriers, in particular focusing on low-income individuals.
Infection with hepatitis B virus (HBV) still constitutes a major public health problem in many countries. More than two billion people have been infected with HBV worldwide, resulting in 240 million chronic carriers. It is estimated that about 780,000 people die annually from complications related to hepatitis B.1 Infection with human immunodeficiency virus (HIV) is also a serious global health problem. About 75 million people have been infected with HIV, with 36 million deaths among individuals with acquired immune deficiency syndrome (AIDS). Globally, there are approximately 35.3 million carriers of HIV.2
Co-infection with HBV and HIV is common.3–5 Both viruses share the same risk factors for infection and can be transmitted through parenteral, sexual, and vertical routes.6 In fact, it is estimated that 10% of HIV carriers in the world have chronic hepatitis B.4 The progression of hepatitis B-associated liver disease is particularly modified by HIV co-infection. In HBV/HIV co-infected individuals, the development of chronic hepatitis B is accelerated, with higher risk of cirrhosis and hepatocellular carcinoma development, and increased morbidity and mortality rates.7–9 Thus, hepatitis B vaccination in the United States of America is recommended by the Advisory Committee on Immunization Practices (ACIP) for all HIV-infected individuals,10 as a strategy to reduce the public health burden caused by co-infection with these two viruses.
Several studies have shown an impaired antibody response to the standard three doses of 20μg HBV vaccine of HBV immunization in HIV-infected patients.11–13 However, a significant higher seroconversion rate was associated with the administration of double dose of the classic 0, 1 and 6-month schedule hepatitis B vaccination among patients with CD4 cell counts ≥350cells/mm3 and HIV viral load <10,000copies/mL.13 A significant increase in the seroconversion rate was also demonstrated in HIV-1 patients using a rapid vaccination schedule of 40μg HBV vaccine at weeks 0, 4, 8 and 24.14 Furthermore, increased compliance with HBV vaccination was verified among HIV-infected patients who received the accelerated schedule.15
In addition to differences in compliance patterns and antibody responses found with the use of different schedules and antigen concentrations, individual factors are also associated with the success of HBV immunization among HIV carriers.16 In this study, the aim was to evaluate the vaccination coverage and immunity against HBV among HIV-infected patients in South Brazil, as well as to identify factors that are associated with vaccination compliance and antibody reactivity in the studied population.
MethodsPatientsThis cross-sectional study was conducted at Professor Polydoro Ernani de São Thiago University Hospital located in Santa Catarina, South Brazil, between October 2012 and March 2013. The sample consisted of HIV-infected men and women receiving free antiretroviral therapy and laboratory monitoring. Universal antiretroviral drugs distribution program was introduced in Brazil in 1996 aiming to ensure free access to essential regimens, including protease inhibitors, for all people living with HIV. In addition, the Brazilian Ministry of Health has established the National CD4+/CD8+ T Lymphocyte Count and Viral Load Laboratory Network to monitor patient therapeutic response.17
This study was approved by the Ethics Committee of the Federal University of Santa Catarina (Protocol 94.398). Informed written consent was obtained from all participants after having received written information.
All the patients in this study were evaluated for their HIV viral load and CD4 cell count. Patients were stratified according to viral load into three groups (<50copies/mL, 50–10,000copies/mL and >10,000copies/mL) and according to their CD4 cell count into two groups (<500cells/μL and ≥500cells/μL). The cut-off of 500cells/mm3 for the CD4 count was taken according to the Brazilian Therapeutic Guidelines for Clinical Management of HIV Infection in Adults.18
The patients answered a self-administered questionnaire, which comprised the following modules: socio-demographic characteristics (including sex, age, ethnicity, and socioeconomic status), transmission route of HIV infection, time since HIV infection diagnosis, and time since initiation of antiretroviral therapy. The surrogate variables for socioeconomic inequalities were monthly income and highest level of education in the household.
The study was conducted in order to determine the vaccination coverage based on the evaluation of vaccination cards and to investigate the association of anti-HBs titers with different socio-demographic aspects. The study sample was divided into three groups (vaccinated, unvaccinated, and possibly unvaccinated) to estimate the effect of the analyzed variables on compliance with vaccination schedules.
Patients who had received the hepatitis B vaccine in the classic three-dose or four double-dose schedules were included in the vaccinated group. Patients vaccinated before the HIV diagnosis received the classic three doses of HBV vaccine. However, it is not possible to know if those patients who had received the three-dose schedule (normal regimen) were HIV-infected at the time of vaccination. On the other hand, patients vaccinated after HIV diagnosis received the four double-dose schedule vaccine.
Patients with no vaccination records who had anti-HBs levels higher than 10.0IU/L and tested negative for HBsAg and/or antibody to hepatitis B core (anti-HBc) serological markers were classified as vaccinated. Those with no vaccination records who tested positive only for anti-HBc were included in the unvaccinated group.
Subjects were also tested for their immune status against HBV, and were classified according to anti-HBs levels: ≤2.0IU/L, 2.1–10.0IU/L, and >10.0IU/L.
AssessmentsA blood sample was collected from each subject for the determination of anti-HBs concentration. After serum separation, anti-HBs antibodies were detected by Chemiluminescence Microparticle Imunoassay (CMIA) using a commercial kit (ARCHITECT®, Abbott Diagnostics, Sligo, Ireland), according to the manufacturer's instructions. CD4 cell count was determined with BD FACSCalibur flow cytometer (Biosciences, San Jose, CA, USA) in units of cells/μL. Plasma HIV RNA was quantified with a branched DNA technique [VERSANT HIV-1 RNA 3.0 (bDNA), Siemens Tarrytown, New York, USA].
Statistical analysisPearson's chi-square test was carried out to examine the association between categorical variables, and a value of p<0.05 was considered significant. All statistical procedures were performed using Statistical Package for Social Science software version 17.0 (SPSS Inc., Chicago, IL, USA).
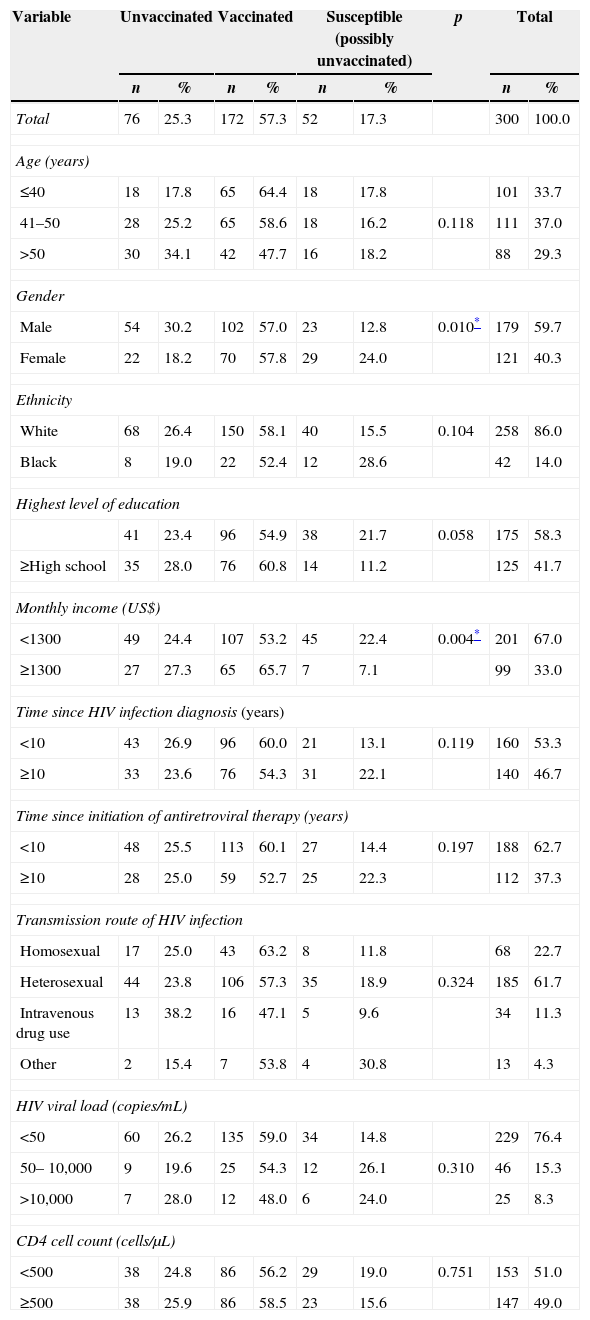
ResultsTable 1 shows the socio-demographic characteristics associated with HBV vaccination coverage among HIV-infected patients in South Brazil. A total of 300 patients were included in the study and out of them, there were 121 (40.3%) women and 179 (59.7%) men. The majority of patients were aged between 41 and 50 years old (37.0%) and the second largest category consisted of younger individuals (≤40 years), comprising 33.7% of the study sample. The older age group (>50 years) comprised 29.3% of the patients (Table 1).
Socio-demographic characteristics associated with HBV vaccination coverage of HIV-infected patients in South Brazil.
| Variable | Unvaccinated | Vaccinated | Susceptible (possibly unvaccinated) | p | Total | ||||
|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | ||
| Total | 76 | 25.3 | 172 | 57.3 | 52 | 17.3 | 300 | 100.0 | |
| Age (years) | |||||||||
| ≤40 | 18 | 17.8 | 65 | 64.4 | 18 | 17.8 | 101 | 33.7 | |
| 41–50 | 28 | 25.2 | 65 | 58.6 | 18 | 16.2 | 0.118 | 111 | 37.0 |
| >50 | 30 | 34.1 | 42 | 47.7 | 16 | 18.2 | 88 | 29.3 | |
| Gender | |||||||||
| Male | 54 | 30.2 | 102 | 57.0 | 23 | 12.8 | 0.010* | 179 | 59.7 |
| Female | 22 | 18.2 | 70 | 57.8 | 29 | 24.0 | 121 | 40.3 | |
| Ethnicity | |||||||||
| White | 68 | 26.4 | 150 | 58.1 | 40 | 15.5 | 0.104 | 258 | 86.0 |
| Black | 8 | 19.0 | 22 | 52.4 | 12 | 28.6 | 42 | 14.0 | |
| Highest level of education | |||||||||
| 41 | 23.4 | 96 | 54.9 | 38 | 21.7 | 0.058 | 175 | 58.3 | |
| ≥High school | 35 | 28.0 | 76 | 60.8 | 14 | 11.2 | 125 | 41.7 | |
| Monthly income (US$) | |||||||||
| <1300 | 49 | 24.4 | 107 | 53.2 | 45 | 22.4 | 0.004* | 201 | 67.0 |
| ≥1300 | 27 | 27.3 | 65 | 65.7 | 7 | 7.1 | 99 | 33.0 | |
| Time since HIV infection diagnosis (years) | |||||||||
| <10 | 43 | 26.9 | 96 | 60.0 | 21 | 13.1 | 0.119 | 160 | 53.3 |
| ≥10 | 33 | 23.6 | 76 | 54.3 | 31 | 22.1 | 140 | 46.7 | |
| Time since initiation of antiretroviral therapy (years) | |||||||||
| <10 | 48 | 25.5 | 113 | 60.1 | 27 | 14.4 | 0.197 | 188 | 62.7 |
| ≥10 | 28 | 25.0 | 59 | 52.7 | 25 | 22.3 | 112 | 37.3 | |
| Transmission route of HIV infection | |||||||||
| Homosexual | 17 | 25.0 | 43 | 63.2 | 8 | 11.8 | 68 | 22.7 | |
| Heterosexual | 44 | 23.8 | 106 | 57.3 | 35 | 18.9 | 0.324 | 185 | 61.7 |
| Intravenous drug use | 13 | 38.2 | 16 | 47.1 | 5 | 9.6 | 34 | 11.3 | |
| Other | 2 | 15.4 | 7 | 53.8 | 4 | 30.8 | 13 | 4.3 | |
| HIV viral load (copies/mL) | |||||||||
| <50 | 60 | 26.2 | 135 | 59.0 | 34 | 14.8 | 229 | 76.4 | |
| 50–10,000 | 9 | 19.6 | 25 | 54.3 | 12 | 26.1 | 0.310 | 46 | 15.3 |
| >10,000 | 7 | 28.0 | 12 | 48.0 | 6 | 24.0 | 25 | 8.3 | |
| CD4 cell count (cells/μL) | |||||||||
| <500 | 38 | 24.8 | 86 | 56.2 | 29 | 19.0 | 0.751 | 153 | 51.0 |
| ≥500 | 38 | 25.9 | 86 | 58.5 | 23 | 15.6 | 147 | 49.0 | |
As shown in Table 1, the most common transmission route of HIV infection was heterosexual contact (61.7%), followed by homosexual contact (22.7%), intravenous drug use (11.3%), and others (4.3%).
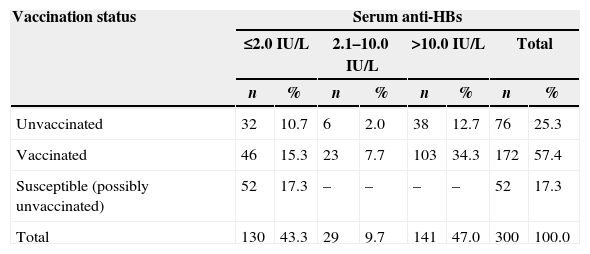
Table 2 shows HBV vaccination coverage and antibody titers among the HIV-infected patients. The overall HBV vaccination coverage in the studied sample was 57.4%, and 56.7% had anti-HBs titers >2.0IU/L; 47.0% had antibody levels higher than 10IU/L (Table 2). The susceptible group (possibly unvaccinated) consisted of patients who did not have a vaccination card at the time of the study, and showed negative HBV serological markers. Among patients who had received hepatitis B vaccine, anti-HBs seropositivity was 73.2% (126/172). Of the 76 unvaccinated individuals, 44 (57.9%) had a positive anti-HBs result. All unvaccinated patients with anti-HBs >2.0IU/L showed a positive test for anti-HBc, a marker of hepatitis B virus infection (Table 2).
Hepatitis B vaccination coverage and antibody titers among the HIV-infected patients.
| Vaccination status | Serum anti-HBs | |||||||
|---|---|---|---|---|---|---|---|---|
| ≤2.0IU/L | 2.1–10.0IU/L | >10.0IU/L | Total | |||||
| n | % | n | % | n | % | n | % | |
| Unvaccinated | 32 | 10.7 | 6 | 2.0 | 38 | 12.7 | 76 | 25.3 |
| Vaccinated | 46 | 15.3 | 23 | 7.7 | 103 | 34.3 | 172 | 57.4 |
| Susceptible (possibly unvaccinated) | 52 | 17.3 | – | – | – | – | 52 | 17.3 |
| Total | 130 | 43.3 | 29 | 9.7 | 141 | 47.0 | 300 | 100.0 |
In this study, anti-HBs was found to be negative (≤2.0IU/L) in 55.4% (67/121) of woman and in 35.2% (63/179) of man. Male gender was significantly associated with high levels of serum antibody (p<0.05). High levels of anti-HBs among HIV-infected patients were also associated with high monthly income (p=0.005). On the other hand, concentration of anti-HBs was not associated with age, CD4 cell count, and HIV viral load (p>0.05).
According to the socio-demographic characteristics of the studied patients shown in Table 1, the HBV vaccination coverage among the females was 57.8% (70/121), whereas 57.0% (102/179) of men had received the vaccine. The majority of the unvaccinated subjects was male and had lower monthly income than the vaccinated individuals (Table 1). On the other hand, women were identified as more likely to be possibly unvaccinated than men (24.0% versus 12.8%, respectively).
Compliance with vaccination against HBV was not associated with age, ethnicity, level of education, time since HIV diagnosis or initiation of antiretroviral therapy, and transmission route of HIV infection (p>0.05) (Table 1).
DiscussionIn Brazil, vaccination against HBV was included in the National Immunization Program in 1996, to high-risk individuals and children younger than one year old. In 2001, the immunization against HBV has been expanded to young people up to 19 years of age.19 Recently, the Brazilian Ministry of Health has again expanded the free vaccination program to individuals up to 49 years old,20 in addition to all HIV-infected patients.21 For HIV carriers, the vaccination schedule of 0, 1, 2 and 6–12 months is recommended, with twice the dose used in the standard protocol.22
The overall coverage of HBV vaccine found in this study (57.4%) was lower than that previously reported among healthy individuals in South Brazil (87.6–97.5%).23–25 This may possibly be consequence of the lack of information concerning hepatitis B vaccination among HIV-infected patients and healthcare professionals. On the other hand, the coverage rate found in this study is similar to the coverage rate (58.2%) estimated among HIV-infected individuals attending 13 centers for HIV care in the United Kingdom.26
In this study, compliance with hepatitis B vaccination schedule was significantly associated with gender. In contrast to other studies on immunization against HBV in HIV carriers,15,27,28 male sex was found to be a predictor of high titers of anti-HBs.
With respect to the immune status, the proportion of subjects with anti-HBs antibody levels higher than 10IU/L reported in the present study (47.0%) is in agreement with that verified among healthy vaccinees of the southern Brazilian region (41.0–58.8%).23–25,29,30 However, it is important to point out that the comparison of results is difficult as the maintenance of anti-HBs titers may be influenced by the time elapsed since vaccination and the level of exposure to hepatitis B virus.31
The rates of HIV-infected individuals who develop anti-HBs levels ≥10IU/L after receiving the standard, accelerated, or four double-dose schedules are variable, ranging from 38.7% to 91.0%.15,16,32 Several studies have shown an association between low-level viremia and better antibody response to HBV vaccination in HIV-infected individuals.12,33,34 In a report comparing the standard and accelerated HBV vaccination schedules among HIV carriers, the use of antiretroviral therapy and HIV-RNA control were associated with enhanced response and development of an anti-HBs titer ≥10IU/L in both vaccination protocols.15
Our results have shown that anti-HBs antibody titers were not significantly associated with CD4 cell count. In agreement with previous observations,35 greater CD4 suppression did not impair antibody reactivity in the studied patients. On the other hand, other findings demonstrated an association between successful immunization against HBV and high CD4 level.11,36 The CD4 cell count has also been reported as a factor associated with antibody responses to different HBV vaccination schedules.16
In the present study, the income disparity was found to be significantly associated with the outcome of hepatitis B vaccination. Although no association was verified with level of education, either the vaccination coverage or anti-HBs titers were lower in HIV-infected individuals with lower monthly income. In addition, the patients living on less than US$ 1300 a month were more likely to be susceptible to HBV infection (possibly non-vaccinated). These data are of concern and emphasize the importance of public health actions directed toward low-income populations. As a result of the lower consumption of health services among poorer populations in Brazil, there is an increase in the framework of social inequality. Low income generates poorer health, and poorer health results in high levels of unemployment and social exclusion.37
In conclusion, our results point toward the need for improvement in the HBV vaccination coverage among HIV carriers in the southern Brazilian region, in particular focusing on low-income individuals. The significant inequality in the coverage rates and antibody reactivity in favor of patients with better economic status highlights the requirement for the development of public strategies in order to increase the availability of healthcare services for poorer population strata. In this regard, the implementation of policies focused on increasing the access to vaccination services might help establishing a successful hepatitis B immunization program among HIV carriers.
Conflicts of interestThe authors declare no conflicts of interest.
This study was supported by the National Council for Scientific and Technological Development (CNPq), Brazil (Process 477160/2011-5).