In recent years, carbapenem-resistant Enterobacteriaceae has become endemic in many countries. Because of limited treatment options, the abandoned “old antibiotics”, polymyxins, have been reintroduced to the clinic. To evaluate the clinical efficacy of polymyxins in the treatment of infections caused by carbapenem-resistant Enterobacteriaceae, we systemically searched the PubMed, Embase, and Cochrane Library databases and analyzed the available evidence. The Preferred Reporting Items for Systematic reviews and Meta-Analysis statement were followed, and the I2 method was used for heterogeneity. Nineteen controlled and six single-arm cohort studies comprising 1086 patients met the inclusion criteria. For controlled studies, no significant difference was noted for overall mortality (OR, 0.79; 95% CI, 0.58–1.08; p=0.15), clinical response rate (OR, 1.24; 95% CI, 0.61–2.54; p=0.55), or microbiological response rate (OR, 0.59; 95% CI, 0.26–1.36; p=0.22) between polymyxin-treated groups and the control groups. Subgroup analyses showed that 28-day or 30-day mortality was lower in patients who received polymyxin combination therapy than in those who received monotherapy (OR, 0.36; 95% CI, 0.19–0.68; p<0.01) and the control groups (OR, 0.49; 95% CI, 0.31–0.75; p<0.01). The results of the six single-arm studies were in accordance with the findings of controlled studies. One controlled and two single-arm studies that evaluated the occurrence of nephrotoxicity reported a pooled incidence rate of 19.2%. Our results suggest that polymyxins may be as efficacious as other antimicrobial therapies for the treatment of carbapenem-resistant Enterobacteriaceae infection. Compared to polymyxin monotherapy, combination regimens may achieve lower 28-day or 30-day mortality. Future large-volume, well-designed randomized control trials are required to determine the role of polymyxins in treating carbapenem-resistant Enterobacteriaceae infections.
In recent years, carbapenemase-producing Enterobacteriaceae, the majority of which is carbapenem-resistant (CRE), have posed a great threat to public health.1 Outbreaks and increased prevalence due to these notorious superbugs have been continuously reported in hospitals worldwide, resulting in high mortality.2 Production of Klebsiella pneumoniae carbapenemase (KPC) enzymes is the most common mechanism of resistance, while the incidence of zinc-dependent metallo-β-lactamases (VIM, IMP, and NDM types) is also increasing.3 The carbapenemase-producing strains can exhibit resistance to most clinically available β-lactams, as well as other important antimicrobial classes such as aminoglycosides and fluoroquinolones.4 Multidrug-resistant (MDR) Enterobacteriaceae make the empiric choice of appropriate antimicrobial treatment very difficult; moreover, the best approach for treating CRE infections is not currently known.
Polymyxins, a group of polypeptide antibiotics, demonstrate potent antimicrobial activity against MDR Gram-negative bacteria by disrupting the outer membrane.5 They were abandoned in the 1960s because of severe adverse effects5; however, limited options for treating infections caused by MDR Gram-negative bacteria have forced clinicians to reuse old drugs.6 So far, many clinical studies have evaluated the efficacy of polymyxins in the treatment of CRE infections, yielding various results. Furthermore, the significant pharmacokinetic deficiencies of polymyxins and the rapid emergence of resistance during treatment have led many clinicians to embrace combination regimens as the preferred treatment strategy for CRE infections.7 Nonetheless, the important question on whether combination therapy, which may increase toxicity and cost, can bring more benefit than monotherapy remains unanswered.
Therefore, in this study, we systemically searched and analyzed the available evidence in order to evaluate the efficacy of polymyxins in the treatment of infections caused by CRE, and to examine whether polymyxin combination therapy can offer an advantage over monotherapy.
MethodsSearch strategiesWe searched the PubMed, Embase, and Cochrane Library databases from their inception until August 30, 2014 using the following search terms: (CRE or carbapenem-resistant or KPC or carbapenemase-producing or VIM or NDM or OXA or IMP) and (Escherichia or Klebsiella or Enterobacter or Proteus or Serratia or Citrobacter or Salmonella or Shigella or Enterobacteriaceae) and (colistin or polymyxin). The references listed in the identified studies were also searched to select relevant articles. No language restrictions were applied. The Preferred Reporting Items for Systematic reviews and Meta-Analysis (PRISMA) statement were used in the identified articles. Two investigators (Ni and Cai) independently performed the literature search and study selection. A third author (Wei) resolved any disagreements, and a final consensus was reached among all authors.
Selection criteriaStudies were considered eligible for inclusion if they provided clinical outcomes of polymyxin therapy for infections caused by carbapenemase-producing Enterobacteriaceae or CRE. For studies reporting the outcomes of both colonized and infected patients, only those of infected patients were extracted. Experimental trials in animals, trials focusing on pharmacokinetic or pharmacodynamic (PK/PD) variables, trials referring only to the in vitro activity of polymyxins, and incomplete unpublished studies were excluded. Case reports and case-series including fewer than 10 infected patients were also excluded.
Ethical considerationsThis study did not require the approval of an ethics committee.
Data extraction and quality assessmentThe following variables were collected from the included studies by two independent reviewers: author, publication year, country, study design, main characteristics and severity of illness (APACHE scores) of the study population, causative pathogens, antibiotic susceptibility testing methods and breakpoints, sites of infections, type and dose of polymyxin administered, coadministration of other antibiotics, outcomes (clinical response, microbiologic eradication, and mortality), and reported toxicity (nephrotoxicity and neurotoxicity).
The quality of the included studies was assessed using the modified Newcastle-Ottawa scale (NOS), which consists of three factors: patient selection, comparability of the study groups, and outcome assessment.8 Studies with a NOS score <3 were classified as having poor quality and were excluded from this systematic review.
Definitions and statistical analysisBecause patients with CRE infections show high mortality rates, we chose mortality as the primary outcome. The secondary outcomes were clinical response, microbiologic eradication, and incidence of toxicity. Because of the lack of standard and uniform criteria for assessing and reporting these secondary outcomes, we accepted the criteria as reported in each study.
All statistical analyses were performed with the Comprehensive Meta-Analysis V2.2 (Biostat, Englewood NJ). The between-study heterogeneity was assessed by using χ2-based Q statistics and the I2 test. Heterogeneity was considered as I2>50%. Either fixed effects (Mantel–Haenszel method) or random effects (DerSimonian and Laird's method) models were used according to the heterogeneity result. Binary outcomes results of controlled studies were expressed as odds ratios (ORs). Egger regression and Begg and Mazumdar methods were used to evaluate publication bias, and p<0.05 was considered statistically significant.
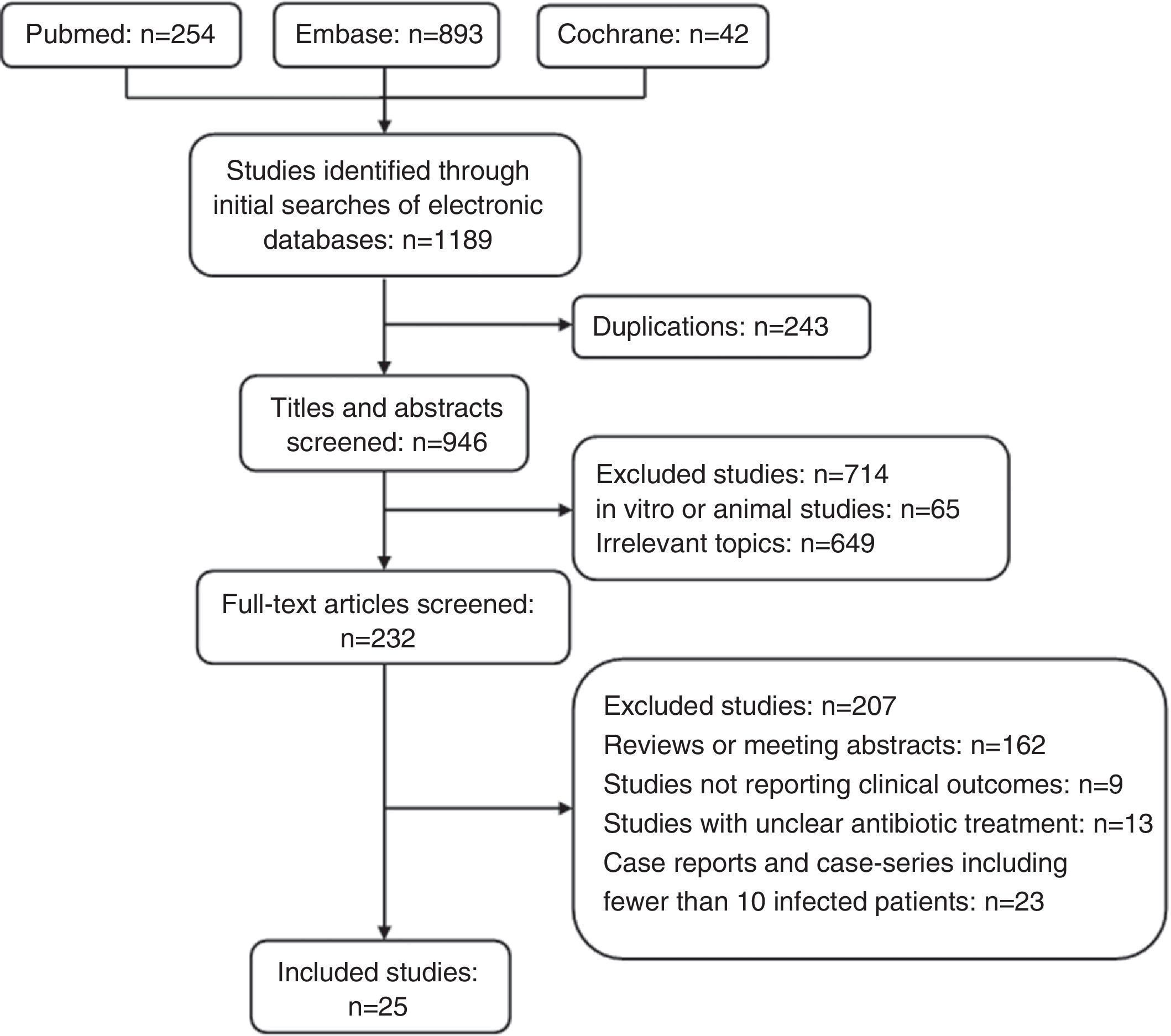
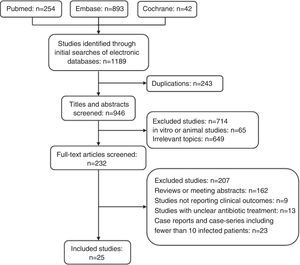
ResultsA total of 1189 potentially relevant references were initially identified by searching the PubMed, Embase, and Cochrane Library databases (Fig. 1). Titles and abstracts were reviewed to exclude irrelevant studies. Two-hundred and thirty-two articles with full texts were screened, and 25 studies met the inclusion criteria.9–33 The examination of the references of these included studies and review articles did not yield any further studies for evaluation. The NOS score of all included studies was >3.
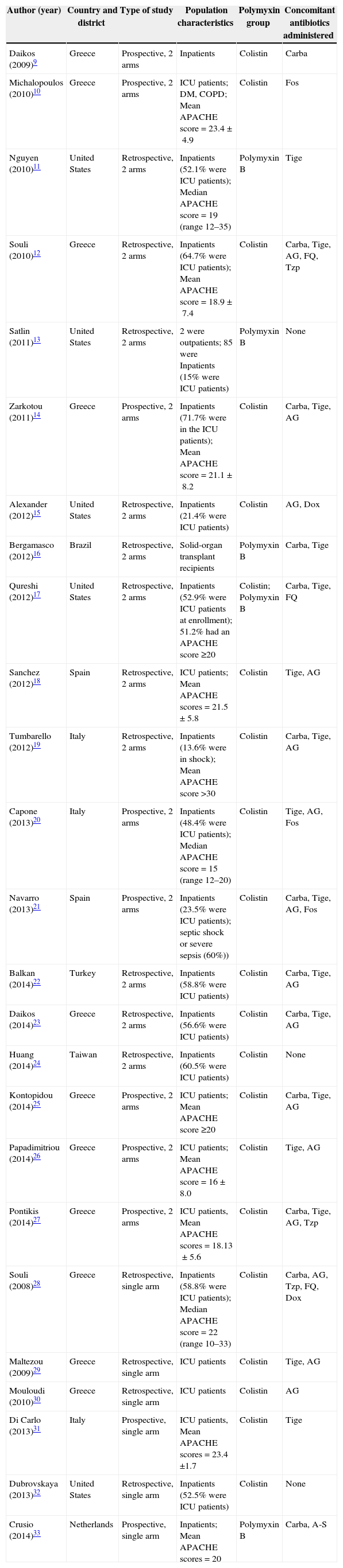
Study characteristicsThe characteristics of the included studies are presented in Table 1. Among the 25 included studies, six involving 175 patients were single-arm studies, and 19 involving 911 patients were controlled studies. Two out of six single-arm studies were prospective studies, and the others were retrospective studies. Eight out of 19 controlled studies were prospective studies, and 11 were retrospective studies. Most patients in the included studies were critically ill. Twenty-two studies involving 881 patients reported that the proportion of patients in the intensive care unit was 64.6%. The average APACHE score of patients in 14 studies using this parameter was >20. Nine studies reported on CRE infections, and 16 other studies reported on carbapenemase-producing Enterobacteriaceae infections. Klebsiella spp. were the major causative pathogen, and bacteremia was the most common manifestation, followed by pneumonia and urinary tract infection.
Characteristics of studies included in systematic review and meta-analysis.
| Author (year) | Country and district | Type of study | Population characteristics | Polymyxin group | Concomitant antibiotics administered |
|---|---|---|---|---|---|
| Daikos (2009)9 | Greece | Prospective, 2 arms | Inpatients | Colistin | Carba |
| Michalopoulos (2010)10 | Greece | Prospective, 2 arms | ICU patients; DM, COPD; Mean APACHE score=23.4±4.9 | Colistin | Fos |
| Nguyen (2010)11 | United States | Retrospective, 2 arms | Inpatients (52.1% were ICU patients); Median APACHE score=19 (range 12–35) | Polymyxin B | Tige |
| Souli (2010)12 | Greece | Retrospective, 2 arms | Inpatients (64.7% were ICU patients); Mean APACHE score=18.9±7.4 | Colistin | Carba, Tige, AG, FQ, Tzp |
| Satlin (2011)13 | United States | Retrospective, 2 arms | 2 were outpatients; 85 were Inpatients (15% were ICU patients) | Polymyxin B | None |
| Zarkotou (2011)14 | Greece | Prospective, 2 arms | Inpatients (71.7% were in the ICU patients); Mean APACHE score=21.1±8.2 | Colistin | Carba, Tige, AG |
| Alexander (2012)15 | United States | Retrospective, 2 arms | Inpatients (21.4% were ICU patients) | Colistin | AG, Dox |
| Bergamasco (2012)16 | Brazil | Retrospective, 2 arms | Solid-organ transplant recipients | Polymyxin B | Carba, Tige |
| Qureshi (2012)17 | United States | Retrospective, 2 arms | Inpatients (52.9% were ICU patients at enrollment); 51.2% had an APACHE score ≥20 | Colistin; Polymyxin B | Carba, Tige, FQ |
| Sanchez (2012)18 | Spain | Retrospective, 2 arms | ICU patients; Mean APACHE scores=21.5±5.8 | Colistin | Tige, AG |
| Tumbarello (2012)19 | Italy | Retrospective, 2 arms | Inpatients (13.6% were in shock); Mean APACHE score >30 | Colistin | Carba, Tige, AG |
| Capone (2013)20 | Italy | Prospective, 2 arms | Inpatients (48.4% were ICU patients); Median APACHE score=15 (range 12–20) | Colistin | Tige, AG, Fos |
| Navarro (2013)21 | Spain | Prospective, 2 arms | Inpatients (23.5% were ICU patients); septic shock or severe sepsis (60%)) | Colistin | Carba, Tige, AG, Fos |
| Balkan (2014)22 | Turkey | Retrospective, 2 arms | Inpatients (58.8% were ICU patients) | Colistin | Carba, Tige, AG |
| Daikos (2014)23 | Greece | Retrospective, 2 arms | Inpatients (56.6% were ICU patients) | Colistin | Carba, Tige, AG |
| Huang (2014)24 | Taiwan | Retrospective, 2 arms | Inpatients (60.5% were ICU patients) | Colistin | None |
| Kontopidou (2014)25 | Greece | Prospective, 2 arms | ICU patients; Mean APACHE score ≥20 | Colistin | Carba, Tige, AG |
| Papadimitriou (2014)26 | Greece | Prospective, 2 arms | ICU patients; Mean APACHE score=16±8.0 | Colistin | Tige, AG |
| Pontikis (2014)27 | Greece | Prospective, 2 arms | ICU patients, Mean APACHE scores=18.13±5.6 | Colistin | Carba, Tige, AG, Tzp |
| Souli (2008)28 | Greece | Retrospective, single arm | Inpatients (58.8% were ICU patients); Median APACHE score=22 (range 10–33) | Colistin | Carba, AG, Tzp, FQ, Dox |
| Maltezou (2009)29 | Greece | Retrospective, single arm | ICU patients | Colistin | Tige, AG |
| Mouloudi (2010)30 | Greece | Retrospective, single arm | ICU patients | Colistin | AG |
| Di Carlo (2013)31 | Italy | Prospective, single arm | ICU patients, Mean APACHE scores=23.4 ±1.7 | Colistin | Tige |
| Dubrovskaya (2013)32 | United States | Retrospective, single arm | Inpatients (52.5% were ICU patients) | Colistin | None |
| Crusio (2014)33 | Netherlands | Prospective, single arm | Inpatients; Mean APACHE scores=20 | Polymyxin B | Carba, A-S |
| Author (year) | Control group | Sample size (Polymyxin group/Control group) | Type of infection | Organisms isolated | Susceptibility testing method (susceptibility breakpoints used) | |
|---|---|---|---|---|---|---|
| Polymyxins | Other antibiotics | |||||
| Daikos (2009)9 | Carba, AG | 23/26 | BSI | VIM-1-producing Klebsiella pneumoniae | Etest (CLSI, 2004) | Etest (CLSI, 2004) |
| Michalopoulos (2010)10 | Fos, AG, Tzp | 6/5 | BSI, VAP, UTI, Wound infection | Carbapenem-resistant K. pneumoniae | NA | NA |
| Nguyen (2010)11 | Tige, other | 22/26 | BSI | Carbapenem-resistant K. pneumoniae | Etest (CLSI, 2009) | Etest; Vitek 2 automated system (CLSI, 2009) |
| Souli (2010)12 | Carba, Tige, AG, Tzp | 14/3 | BSI, SSI, UTI, HAP | KPC-producing K. pneumoniae | Etest (EUCAST, 2009) | Etest; Agar dilution (CLSI, 2009; FDA) |
| Satlin (2011)13 | Tige, AG | 25/62 | UTI | Carbapenem-resistant K. pneumoniae | Etest (CLSI, 2011) | Etest; Vitek 2 automated system (CLSI, 2011; FDA) |
| Zarkotou (2011)14 | Carba, Tige, AG | 21/14 | BSI | KPC-producing K. pneumoniae | Broth microdilution (EUCAST, 2010) | Vitek 2 automated system; Broth microdilution (CLSI, 2010) |
| Alexander (2012)15 | AG, Dox, FQ, Ntf | 2/12 | UTI, BSI | KPC-producing K. pneumoniae | Disk diffusion (CLSI, 2006) | Disk diffusion; Etest (CLSI, 2006) |
| Bergamasco (2012)16 | Carba, Tige | 9/3 | BSI, UTI, SSI, HAP | KPC-producing K. pneumoniae | Etest (CLSI, 2009) | Disk diffusion; Etest (CLSI, 2009; FDA) |
| Qureshi (2012)17 | Carba, Tige, AG, FQ, Azt, Cfpm, Tzp, A-S | 14/20 | BSI | KPC-producing K. pneumoniae | Broth microdilution (CLSI, 2011) | Broth microdilution; Etest (CLSI, 2011) |
| Sanchez (2012)18 | Carba, Tige, AG | 12/12 | Pneumonia, LRTI, UTI, Meningitis, BSI, IAI, SSTI | VIM-1-producing K. pneumoniae | Broth microdilution; Etest (CLSI, 2011) | Broth microdilution; Etest (CLSI, 2011; EUCAST, 2011) |
| Tumbarello (2012)19 | Carba, Tige, AG | 61/36 | BSI | KPC-producing K. pneumoniae | Vitek 2 automated system (CLSI, 2011) | Vitek 2 automated system (CLSI, 2011; FDA) |
| Capone (2013)20 | Tige, AG, Fos | 36/22 | BSI, UTI, Septic shock, LRTI, SSTI | KPC-producing K. pneumoniae | Broth microdilution (EUCAST, 2010) | Broth microdilution (EUCAST, 2010) |
| Navarro (2013)21 | Carba, Tige, AG, Fos, FQ, Cef | 18/16 | BSI | OXA-48-producing Enterobacteriaceae (K. pneumoniae, Escherichia coli) | Etest (CLSI, 2012) | Vitek 2 automated system; Etest (CLSI, 2012; FDA) |
| Balkan (2014)22 | Carba, Tige, AG | 24/12 | BSI | OXA-48-producing Enterobacteriaceae (K. pneumoniae, E. coli, Enterobacter aerogenes) | Etest (EUCAST, 2013) | Etest (EUCAST, 2013) |
| Daikos (2014)23 | Carba, Tige, AG, other | 78/86 | BSI | Carbapenem-Resistant K. pneumoniae | Etest (EUCAST, 2013) | Etest; Vitek 2 automated system (EUCAST, 2013) |
| Huang (2014)24 | Carba, Tige | 4/29 | NA | Carbapenem-resistant Enterobacteriaceae (K. pneumoniae, E. coli) | Broth microdilution (EUCAST, 2012) | Broth microdilution (EUCAST, 2012) |
| Kontopidou (2014)25 | Tige, AG, FQ | 57/50 | VAP, UTI, BSI, SSI, IAI | Carbapenem-Resistant K. pneumoniae | Vitek 2 automated system (EUCAST, 2012) | Etest; Vitek 2 automated system (CLSI, 2010; EUCAST, 2012) |
| Papadimitriou (2014)26 | Tige, AG | 19/17 | BSI | KPC-producing K. pneumoniae | Etest (CLSI, 2011) | Etest, Disk diffusion (CLSI, 2011) |
| Pontikis (2014)27 | Tige, AG | 10/5 | BSI, UTI, VAP, IAI, Meningitis | Carbapenem-Resistant K. pneumoniae | Vitek 2 automated system (CLSI, 2012) | Vitek 2 automated system (CLSI, 2012; FDA) |
| Souli (2008)28 | NA | 16/NA | BSI; VAP | VIM-1, MBL producing Enterobacteriaceae (Klebsiella spp., Enterobacter spp.) | Etest (BSAC) | Disk; Etest (CLSI, 2006) |
| Maltezou (2009)29 | NA | 11/NA | Pneumonia, SSI | KPC-2-producing K. pneumoniae | Etest (CLSI, 2007) | Disk; Etest (CLSI, 2007) |
| Mouloudi (2010)30 | NA | 53/NA | BSI | KPC, MBL producing K. pneumoniae | Etest (EUCAST, 2010) | Etest, Broth microdilution (CLSI, 2007; FDA) |
| Di Carlo (2013)31 | NA | 30/NA | SSI, IAI, | KPC-producing K. pneumoniae | Etest (EUCAST, 2013) | Broth microdilution (EUCAST, 2013) |
| Dubrovskaya (2013)32 | NA | 40/NA | BSI, UTI, SSI, Pneumonia, IAI | Carbapenem-Resistant K. pneumoniae | Etest (CLSI, 2012) | Etest; Vitek 2 automated system (CLSI, 2012; FDA) |
| Crusio (2014)33 | NA | 25/NA | BSI, VAP, UTI | Carbapenem-Resistant K. pneumoniae | Vitek 2 automated system (CLSI, 2009) | Vitek 2 automated system (CLSI, 2009) |
Abbreviation: NA, not applicable; ICU, intensive care unit; BSI, bloodstream infection; VAP, ventilator-associated pneumonia; UTI, urinary tract infection; SSI, surgical-site infection; HAP, hospital-acquired pneumonia; LRTI, lower respiratory tract infection; IAI, intra-abdominal infection; SSTI, skin and soft tissue infection. Carba, carbapenem; Tige, tigecycline; Ntf, nitrocefin; Fos, fosfomycin; AG, aminoglycoside; A-S, ampicillin-sulbactam; Azt, aztreonam; FQ, fluoroquinolone; Caz, ceftazidime; Cfpm, cefepime; Cef, Ceftriaxone; Tzp, piperacillin-tazobactam; Dox, doxycycline; CLSI, Clinical and Laboratory Standards Institute; EUCAST, European Committee on Antimicrobial Susceptibility Testing; FDA, Food and Drug Administration; BSAC, British Society for Antimicrobial Chemotherapy.
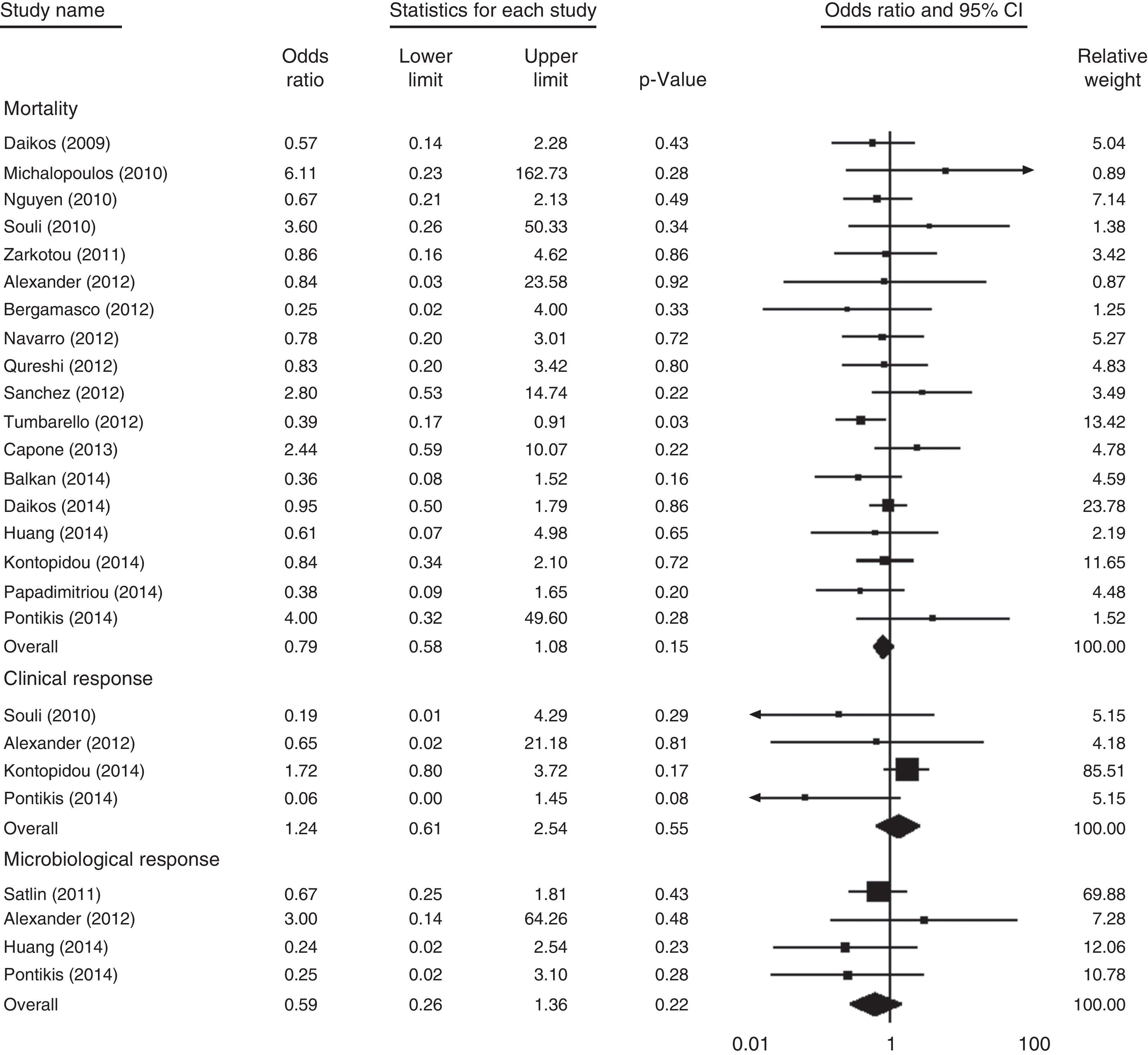
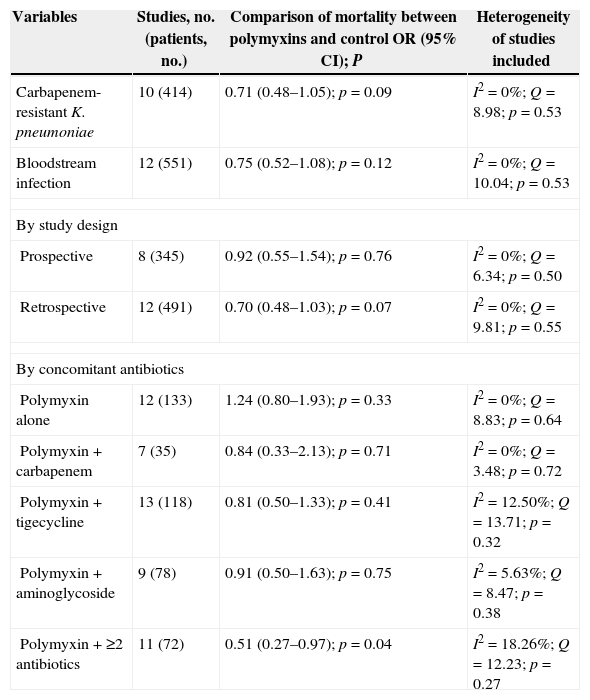
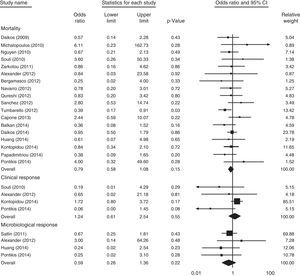
Among the 19 controlled studies, 18 involving 824 patients reported the mortality rate. As shown in Fig. 2, no significant difference in overall mortality was noted when the polymyxins-treated groups were compared with the control groups (OR, 0.79 [95% confidence interval (CI), 0.58–1.08; p=0.15]; I2=0%; Q=15.12 [p=0.59]). The subgroup analysis of controlled studies is presented in Table 2.
Subgroup analysis of overall mortality with polymyxin-based therapy versus control antibiotics for treatment of carbapenem-producing Enterobacteriaceae and carbapenem-resistant Enterobacteriaceae infections in controlled studies.
| Variables | Studies, no. (patients, no.) | Comparison of mortality between polymyxins and control OR (95% CI); P | Heterogeneity of studies included |
|---|---|---|---|
| Carbapenem-resistant K. pneumoniae | 10 (414) | 0.71 (0.48–1.05); p=0.09 | I2=0%; Q=8.98; p=0.53 |
| Bloodstream infection | 12 (551) | 0.75 (0.52–1.08); p=0.12 | I2=0%; Q=10.04; p=0.53 |
| By study design | |||
| Prospective | 8 (345) | 0.92 (0.55–1.54); p=0.76 | I2=0%; Q=6.34; p=0.50 |
| Retrospective | 12 (491) | 0.70 (0.48–1.03); p=0.07 | I2=0%; Q=9.81; p=0.55 |
| By concomitant antibiotics | |||
| Polymyxin alone | 12 (133) | 1.24 (0.80–1.93); p=0.33 | I2=0%; Q=8.83; p=0.64 |
| Polymyxin+carbapenem | 7 (35) | 0.84 (0.33–2.13); p=0.71 | I2=0%; Q=3.48; p=0.72 |
| Polymyxin+tigecycline | 13 (118) | 0.81 (0.50–1.33); p=0.41 | I2=12.50%; Q=13.71; p=0.32 |
| Polymyxin+aminoglycoside | 9 (78) | 0.91 (0.50–1.63); p=0.75 | I2=5.63%; Q=8.47; p=0.38 |
| Polymyxin+≥2 antibiotics | 11 (72) | 0.51 (0.27–0.97); p=0.04 | I2=18.26%; Q=12.23; p=0.27 |
Abbreviations: OR, odds ratio; CI, confidence interval.
For the six single-arm studies, the pooled overall mortality rate was 35.7% (95% CI, 0.22–0.53; I2=74.85%; Q=19.88 [p=0.001]), which was in line with the results of the controlled studies (33.8% [95% CI, 0.29–0.39]; I2=27.43%; Q=23.43 [p=0.14]).
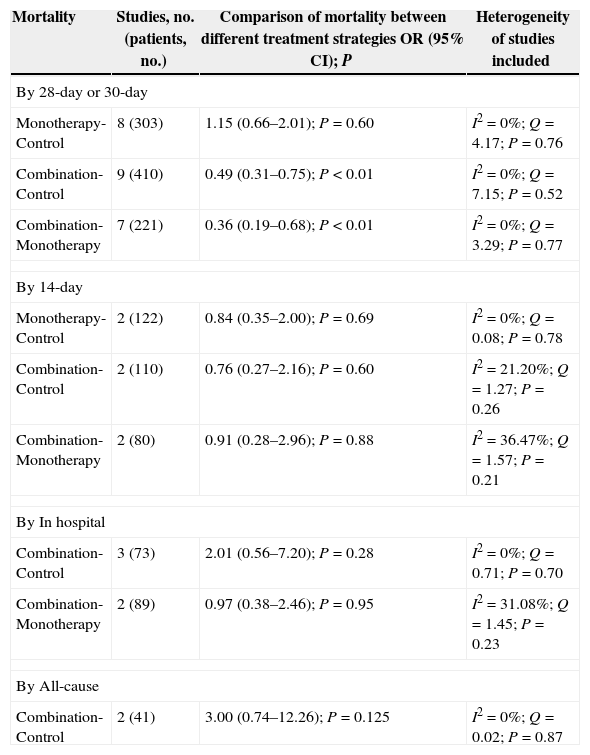
The subgroup analysis by mortality type is shown in Table 3. With respect to 28-day or 30-day mortality, no significant difference was observed between the polymyxin monotherapy and control groups, while significantly lower mortality was noted in the combination therapy group. For other subgroup analyses, such as in-hospital mortality, 14-day mortality, and all-cause mortality, the rate did not differ significantly between the polymyxin-treated groups and the control groups. In addition, one study compared colistin with other antibiotics in terms of infection-related mortality, and no significant difference was found between the two groups.
Subgroup analysis of mortality with different polymyxin treatment strategies in the treatment of carbapenem-producing Enterobacteriaceae and carbapenem-resistant Enterobacteriaceae infections.
| Mortality | Studies, no. (patients, no.) | Comparison of mortality between different treatment strategies OR (95% CI); P | Heterogeneity of studies included |
|---|---|---|---|
| By 28-day or 30-day | |||
| Monotherapy-Control | 8 (303) | 1.15 (0.66–2.01); P=0.60 | I2=0%; Q=4.17; P=0.76 |
| Combination-Control | 9 (410) | 0.49 (0.31–0.75); P<0.01 | I2=0%; Q=7.15; P=0.52 |
| Combination-Monotherapy | 7 (221) | 0.36 (0.19–0.68); P<0.01 | I2=0%; Q=3.29; P=0.77 |
| By 14-day | |||
| Monotherapy-Control | 2 (122) | 0.84 (0.35–2.00); P=0.69 | I2=0%; Q=0.08; P=0.78 |
| Combination-Control | 2 (110) | 0.76 (0.27–2.16); P=0.60 | I2=21.20%; Q=1.27; P=0.26 |
| Combination-Monotherapy | 2 (80) | 0.91 (0.28–2.96); P=0.88 | I2=36.47%; Q=1.57; P=0.21 |
| By In hospital | |||
| Combination-Control | 3 (73) | 2.01 (0.56–7.20); P=0.28 | I2=0%; Q=0.71; P=0.70 |
| Combination-Monotherapy | 2 (89) | 0.97 (0.38–2.46); P=0.95 | I2=31.08%; Q=1.45; P=0.23 |
| By All-cause | |||
| Combination-Control | 2 (41) | 3.00 (0.74–12.26); P=0.125 | I2=0%; Q=0.02; P=0.87 |
Abbreviations: OR, odds ratio; CI, confidence interval.
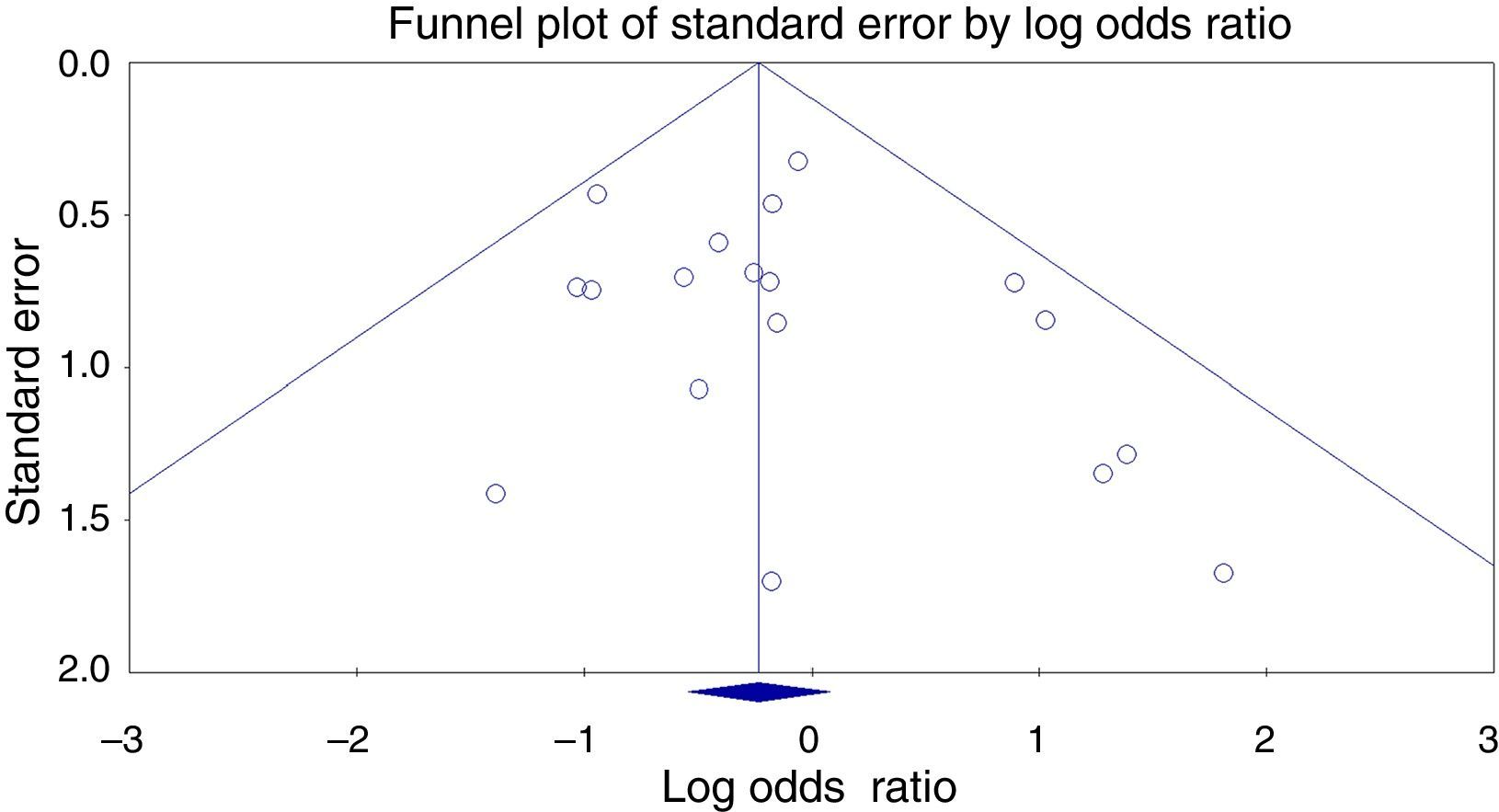
No publication bias was detected by using Egger regression or Begg and Mazumdar rank correlation. Therefore, the funnel plot for publication bias demonstrated no marked evidence of asymmetry, as shown in Fig. 3.
Clinical responseFour studies involving 153 patients compared the clinical response of colistin-based therapy with that of other antibiotic regimens. As shown in Fig. 2, no significant difference was observed between the two groups (OR, 1.24 [95% CI, 0.61–2.54; p=0.55]; I2=47.45%; Q=5.71 [p=0.13]). For the single-arm studies (three studies; 81 patients), the favorable clinical response was 75% (95% CI, 0.64–0.83; I2=0%; Q=1.50 [p=0.47]). One study compared colistin combination therapy (13 of 31 patients) with monotherapy (8 of 26 patients), and no significant difference was found between the two groups (p=0.55).
Microbiological responseAs shown in Fig. 2, four studies involving 149 patients reported the outcome of microbiological response. No significant difference was noted between the polymyxin-treated groups and the control groups (OR, 0.59 [95% CI, 0.26–1.36; p=0.22]; I2=0%; Q=2.17 [p=0.54]). In three single-arm studies (62 patients), the overall microbiological response rate was 51% (95% CI, 0.38–0.63; I2=28.30%; Q=2.79 [p=0.25]).
Adverse eventsNeurotoxicity was not reported in any of the studies. Only one controlled study and two single-arm studies evaluated the occurrence of nephrotoxicity. Pooled analysis showed an incidence rate of 19.2% (95% CI, 0.08–0.39; I2=67.12%; Q=6.08 [p=0.05]).
DiscussionCarbapenemase-producing Enterobacteriaceae, particularly KPC-producing K. pneumoniae, is now widespread and endemic in many countries.34 Infections caused by these MDR organisms are associated with high treatment failure and mortality.35 However, available effective therapeutic options are scarce. In this situation, polymyxins, which exhibit potent in vitro activity against MDR Gram-negative bacteria, have recently become the focus of interest to clinicians.36 Nevertheless, before polymyxins were widely reintroduced in the clinic, comprehensive and objective evaluation of these “old antibiotics” was of great necessity.
In this systemic review, we assessed the available evidence for the efficacy of polymyxins in treating CRE infections. Although no statistical difference was observed, a strong tendency toward lower mortality was noted in the polymyxin-treated groups (OR, 0.79; 95% CI, 0.58–1.08). As for the clinical and microbiological response, pooled data showed no significant difference between the two groups. Therefore, we can at least conclude that polymyxins are as efficacious as other antibiotics for treating CRE infections.
Nevertheless, the rapid emergence of resistant isolates and suboptimal pharmacokinetics may challenge the efficacy of polymyxins in treating MDR infections, especially bacteremia and pneumonia.37,38 Many clinicians believe that combination therapy may overcome these shortcomings. Prospective studies showed that polymyxin-based combination therapy could result in better clinical and microbiological outcomes than monotherapy, but failed to provide evidence for the superiority of combination therapy in lowering mortality when treating MDR Acinetobacter baumannii infections.39–41 The role of combination therapy in CRE infections has not been well evaluated. In the subgroup analyses of our study, polymyxin monotherapy did not lower the 28-day or 30-day mortality, and the outcome was in favor of the control groups. In contrast, combination therapy significantly lowered the 28-day or 30-day mortality. This indicates that polymyxin combination therapy may have an advantage over monotherapy in treating CRE infections, although the evidence is not strong enough.
Several important questions regarding combination therapy remain unanswered, such as the best combination for each infection type, the continued role for carbapenems in combination therapy, and the timing of combination therapy initiation.7 A number of in vitro synergy tests have been performed to verify the synergistic effects of polymyxins in combination with other antibiotics.42–44 However, the significant synergy observed in vitro should be carefully interpreted, because the PK/PD effects of drugs in vivo, bacterial load, and drug concentrations in specific sites of infection are different.45 A recent study found that when the minimum inhibitory concentrations (MICs) for carbapenems were ≤8mg/L, carbapenem-containing regimens seemed to offer therapeutic advantage over other regimens.46 However, for Enterobacteriaceae with MICs for carbapenems >8mg/L, a combination of two or even three antibiotics, such as colistin, high-dose tigecycline, aminoglycoside, and fosfomycin, seemed to decrease mortality.46 In this study, subgroup analyses revealed that polymyxins combined with carbapenems, tigecycline, or aminoglycosides could not significantly lower mortality; only triple polymyxin-containing combinations seemed to do so. Considering the limited number of patients and potential bias existing in the included studies, we cannot draw definitive conclusions. Much research is needed in the future to address these significant questions.
The biggest limitation to the wider clinical application of polymyxins is the dosing-related nephrotoxicity and neurotoxicity. Among the included studies, only one controlled study and two single-arm studies evaluated the occurrence of nephrotoxicity, and pooled analysis showed an incidence rate of 19.2%. No studies have reported any incidences of neurotoxicity, such as seizures, encephalopathy, and neuromuscular blockade. Owing to the limited available data, we were unable to compare the safety between the polymyxin-treated groups and the control groups. Recent studies report less frequent and severe adverse effects than that reported in the 1970s.47 Other published systemic reviews have concluded that the administration of polymyxins was not associated with a relatively higher incidence of nephrotoxicity.36,41 Possible reasons might be improved purified drug formulations, careful dosing, close renal function monitoring, and more advanced critical care services.38 However, renal function should always be closely monitored during administration, and the clinical safety of polymyxins requires further investigation.
Our study should be interpreted with caution, as it has limitations that must be taken into account. The main limitation is that none of the included studies were prospective randomized controlled trials (RCTs). Therefore, we were unable to control for some confounding factors such as different patient populations, different sites of infections, different genotypes of pathogens, and different antimicrobial susceptibility breakpoints. Another limitation is that most of the included studies did not provide sufficient detail to facilitate the comprehensive interpretation of the results, such as the MICs, total daily doses, time to initiate therapy, and duration of therapy. In addition, the sample size for specific subgroup analysis was small, which may reduce the power of statistical analyses.
In summary, this systematic review and meta-analysis indicates that polymyxins and other antimicrobial therapies may have similar efficacy in the treatment of infections caused by carbapenemase-producing Enterobacteriaceae and CRE. Compared with polymyxin monotherapy, combination regimens may achieve lower 28-day or 30-day mortality. However, the inherent limitations of the included studies prevent us from reaching definitive conclusions. Future large-volume, well-designed RCTs are required to determine the role of polymyxins in treating CRE infections.
FundingThis work was supported by the National Natural Science Foundation of China (No. 81371855). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflicts of interestThe authors declare no conflicts of interest.