Antiretroviral therapy has significantly evolved in the last decade, with an increasing number of new drugs and classes. Currently, even heavily experienced patients can be successfully treated with new regimens. In Brazil, the recent incorporation of some new antiretroviral drugs made it possible to suppress HIV plasma viremia in most treated patients, with significant benefits in terms of quality of life and survival. However, little has been published on outcomes of patients under new drugs-based regimens. We reviewed the safety and efficacy of antiretroviral regimens using recently introduced drugs in Bahia. Our results confirm that patients using darunavir, raltegravir, enfuvirtide, or etravirine presented with a high rate of virological suppression without significant adverse events, after one year of follow-up.
One of the most important problems in the treatment of HIV-1 infected patients has been the development of resistance to antiretroviral (ARV) drugs. In the early HAART era, patients exposed to multiple ARV regimens usually presented with a broad spectrum of drug resistance, making it difficult to define a fully suppressive ARV regimen. The absence of effective treatment usually led to disease progression and death.1 The development of new drugs and classes of ARV dramatically modified the scenario of salvage therapy for patients previously exposed to multiple regimens. Several studies provided strong evidence that even patients with complex drug resistance profile could be successfully treated with a regimen that include new, active ARV drugs.2–6
Brazil recently provided access to new ARV drugs like ritonavir-boosted darunavir (DRV/r), raltegravir (RAL), etravirine (ETV), and maraviroc (MRV). However, the Brazilian AIDS Program established several rules to use these new ARV drugs, which are available only as “third line” options. There is scarce information on the outcomes of patients treated with these new drugs/classes in developing countries. In the present work we evaluated the impact of new drugs use on viral suppression, and its tolerability by patients with a history of previous failures to multiple regimens.
We reviewed the Bahia state's central pharmacy registry to identify the first patients who used darunavir/ritonavir (DRV/r), enfuvirtide (ENF), raltegravir (RAL), etravirine (ETV) or maraviroc (MVC) up to August 2011. After identification of all patients who used or were in use of these ARV drugs, we reviewed the medical records to evaluate clinical characteristics, virological response, and tolerability of ARV regimens containing these new drugs.
We compared CD4 counts and plasma HIV-1 RNA viral loads at the moment of initiation of ARV regimens containing one or more of these new drugs, and 6 and 12 months thereafter. The proportion of patients with undetectable viral load was compared at each time-point, as well as the change in CD4 count. Adherence to therapy was also evaluated, and the frequency of adverse events was recorded.
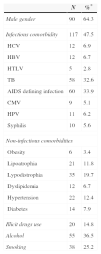
Mean age for patients included in this study was 41.2±11.9 years. Around two-thirds (60%) were male, and black/racially mixed (68%). Only 17% described themselves as white, while 15% were of other ethnic groups. The mean number of previous ARV regimens was 4.8±3.3. Mean HIV-1 RNA plasma viral load was 4.6±1.3logcopies/mL, while mean CD4+ cell count was 273±32cells/mm3. As expected in a subpopulation of patients with long-term treatment history, most of them had a previous diagnosis of infectious and non-infectious comorbidities. Table 1 shows the main health problems detected in this population.
Clinical characteristics of 194 patients who started an ARV regimen containing new ARV drugs.
| N | %* | |
|---|---|---|
| Male gender | 90 | 64.3 |
| Infectious comorbidity | 117 | 47.5 |
| HCV | 12 | 6.9 |
| HBV | 12 | 6.7 |
| HTLV | 5 | 2.8 |
| TB | 58 | 32.6 |
| AIDS defining infection | 60 | 33.9 |
| CMV | 9 | 5.1 |
| HPV | 11 | 6.2 |
| Syphilis | 10 | 5.6 |
| Non-infectious comorbidities | ||
| Obesity | 6 | 3.4 |
| Lipoatrophia | 21 | 11.8 |
| Lypodistrophia | 35 | 19.7 |
| Dyslipidemia | 12 | 6.7 |
| Hypertension | 22 | 12.4 |
| Diabetes | 14 | 7.9 |
| Illicit drugs use | 20 | 14.8 |
| Alcohol | 55 | 36.5 |
| Smoking | 38 | 25.2 |
We identified a total of 546 medical prescriptions of at least one of these new ARV, distributed between the four referral centers in Salvador city, Bahia, Brazil, and several smaller cities in Bahia. Table 2 summarizes the number of prescriptions of each ARV drug. Due to the limited access to medical charts from other institutions, we focused the review on medical records of two main referral centers for AIDS care in Bahia, namely Centro de Ensino, Diagnóstico, Assistencia e Pesquisa (CEDAP) and Complexo Hospitalar Prof. Edgard Santos (C-HUPES), at the Federal University of Bahia Hospital. Together, these two institutions concentrate about two-thirds of the total number of patients in the state. Thus, a total of 246 patients had their records selected for review. However, 52 of them were excluded, because their medical charts were incomplete, with many missing information. The remaining 194 (79%) patients were included in this analysis. Of note, only 15 (6%) patients were in use of three new ARV (DRV/r+RAL+ETV in nine cases, RAL+DRV/r+ENF in six cases).
Number of medical prescriptions for new drugs in the study's period.
| Drug | Number of prescriptions for each drug | ||||
|---|---|---|---|---|---|
| RAL (%) | DRV/R (%) | ENF (%) | ETV (%) | Total | |
| RAL | 143 (51.8) | 111 (40.2) | 17 (6.1) | 5 (1.8) | 276 (100) |
| DRV/r | 111 (40.2) | 79 (33.3) | 06 (2.8) | 06 (2.8) | 212 (100) |
| ENF | 17 (46.3) | 6 (15.4) | 16 (41.0) | 0 | 39 (100) |
| ETV | 05 (26.3) | 6 (31.6) | 0 | 8 (42.1) | 19 (100) |
| Total | 276 (50.5) | 212 (38.8) | 39 (7.1) | 19 (3.5) | 546 (100) |
After six months of therapy with the new drugs 71.1% of patients had HIV-1 RNA plasma viral load below the limit of detection (50copies/mL), and after 12 months this rate increased to 74.7%. Mean CD4+ cell count at month six was 377±274cells/mm3 (net gain from baseline of 104cells/m3), raising to 476±282cells after 12 months (additional gain of 99cells/mm3, p<0.001 for both values, compared to baseline). Mean HIV-1 RNA plasma viral load (log10copies/mL) was 4.6±1.3 at baseline, decreased to 1.7±1.2 at six months, and to 1.7±1.2 at 12 months (p<0.001 for comparison with baseline values for both time-points). Table 3 details laboratory characteristics of patients at diagnosis, at therapy baseline, and 6/12 months after salvage regimen had been introduced.
Mean HIV-1 RNA plasma viral load (PVL) and CD4/8 count at baseline, pre-therapy, and 6/12 months post therapy with new drugs.
| Mean | SD | Minimum | Maximum | Percentiles | |||
|---|---|---|---|---|---|---|---|
| 25 | Median | 75 | |||||
| First evaluation (at diagnosis) | |||||||
| PLV (log10) | 4.73 | 1.16 | 1.00 | 6.99 | 4.09 | 4.88 | 5.56 |
| CD4 (cells/mm3) | 276 | 320 | 4 | 2140 | 56 | 168 | 334 |
| CD8 (cells/mm3) | 966.9 | 587.6 | 51 | 2556 | 458 | 854 | 1345 |
| Evaluation before introduction of new drugs | |||||||
| PVL (log10) | 3.67 | 1.65 | 1.00 | 6.00 | 2.41 | 4.30 | 4.93 |
| CD4 (cells/mm3) | 377 | 274 | 2 | 1300 | 101 | 242 | 429 |
| CD8 (cells/mm3) | 938.8 | 599.2 | 108 | 2839 | 514 | 784 | 1234 |
| Evaluation after 6 months under new drugs regimen | |||||||
| PVL (log10) | 1.73 | 1.31 | 1.00 | 5.64 | 1.00 | 1.00 | 1.99 |
| CD4 (cells/mm3) | 394.2 | 247.7 | 19 | 1270 | 212 | 338 | 530 |
| CD8 (cells/mm3) | 1145.8 | 590.4 | 72 | 3018 | 761 | 1065 | 1375 |
| Evaluation after 12 months under new drugs regimen | |||||||
| PVL (log10) | 1.81 | 1.44 | 1.00 | 5.29 | 1.00 | 1.00 | 2.54 |
| CD4 (cells/mm3) | 448.0 | 272.9 | 2 | 1592 | 295 | 398 | 569 |
| CD8 (cells/mm3) | 1263.6 | 601.0 | 133 | 2958 | 782 | 1182 | 1777 |
p<0.05 for comparisons between CD4 and viral load at baseline (time of regimen change) and either 6 months/12 months values.
The main adverse events reported by patients are summarized in Table 4. Of note, gastrointestinal complaints were the most commonly detected problems: nausea or diarrhea was reported by 10.7% of patients. Local pain, the most frequent adverse event related to ENF, was detected in 20/39 (64%) of patients on ENF. Optimal (100%) self-reported adherence rate to new ARV regimen was 71%. Laboratory parameters were similar after six and 12 months, compared to baseline values, except for creatinine (variation from 0.9 to 1.0 after six months and from 1.0 to 1.1 after 12 months, (p<0.01 for comparison of both time-points with baseline), and for HDL cholesterol, which showed a mean decrease of 4mg at six and 12 months when compared to baseline values (p=0.04). No significant variations were observed in liver enzymes, lipids other than HDL cholesterol, hemoglobin or glucose.
Frequency of adverse events among patients in use of new antiretroviral drugs.
| Adverse events (>3%) | N | % |
|---|---|---|
| Nausea/vomiting | 13 | 10.7 |
| Diarrhea | 13 | 10.7 |
| Local pain | 25 | 20.3 |
| Headache | 10 | 8.2 |
| Astenia | 4 | 3.3 |
| Myalgia | 6 | 4.9 |
| Dizziness | 6 | 4.9 |
| Sleep changes | 4 | 3.3 |
| Other | 45 | 23.2 |
| Optimal adherence (self-report) | 63 | 71.6 |
Forty-seven patients (19%) had their ARV regimens changed, after starting a regimen containing at least one new drug. In 21 cases, switch was motivated by adverse events, but virological failure also contributed to regimen change in 20 patients. The remaining six patients had discontinued therapy for other reasons.
Virological response to ART regimens including new antiretroviral drugs/classes for salvage therapy was very high (72%), as evaluated after six months of therapy. This high response rate even increased (79%) after 12 months of follow-up, demonstrating great virological efficacy in heavily experienced patients. Moreover, the new regimens were well tolerated, and adherence level was high, which certainly contributed to these responses.
Most of the patients received ARV regimens based on the use of RAL and/or DRV/r, a minority being treated with regimens including ETV or ENF. DRV/r plus RAL was the most (58%) used regimen, with/without a third new drug. The main side-effect observed was reaction at the local of ENF injection, followed by gastrointestinal symptoms (nausea/vomiting or diarrhea).
The initial regimens used for salvage therapy were based in only one new, active drug (usually a PI), and resulted in modest outcomes, as it was observed in TORO (ENF), POWER (DRV/r) and RESIST (TPV/r) trials.6–9 Availability of more recent ARV dramatically changed this scenario. The BENCHMARK trial provided rates of up to 80% success at six months, when at least two new drugs were used.6 Also, there has been a previous trial showing high response rates with DRV/r and ETV, and recent studies showing rates close to 100% in salvage therapy of patients treated with a combination of three new drugs, from different classes.10–12
In Brazil, three previous studies evaluated the effectiveness of DRV/r-based salvage regimens for highly experienced patients and found similar results after 48 weeks. Vidal et al. prospectively followed 92 patients who received a DRV/r-based ARV salvage regimen, and reported 83% virological suppression rate.13 Another group published two additional papers, with a similar approach with rates of virological suppression of 73 and 78%.14,15 All of these studies followed a common strategy (DRV/r-based regiments plus optimized backbone) with very close results to those found in our study.
Currently, the availability of a large number of ARV drugs makes it possible to choose the best regimen for treating experienced patients failing therapy, with a high likelihood of sustainable virological response. In addition, the newer classes have also a very good safety profile, reducing the risks of lower adherence/lower response due to adverse events.11
In conclusion, the real life experience here described demonstrates that virological response of heavily pre-treated patients can be as high as that found in clinical trials. The use of newer drugs and classes makes it easier to treat such patients, who present with highly resistant HIV-1 strains, in comparison to the early HAART era. In addition, the favorable safety profile of these drugs ensures a high level of adherence to therapy, with no significant clinical or laboratory abnormalities.
Conflicts of interestThe authors declare no conflicts of interest.
This study was supported by a grant from Departamento de DST/AIDS e Hepatites Virais, Ministério da Saúde, Brasil/UNESCO.