In coinfected HBV/HCV patients, HBV replication is usually suppressed by HCV over the time. No study to date has evaluated the HBV viremia in long-term follow-up after HCV treatment in hemodialysis patients with HBV/HCV coinfection. This study aimed to assess the evolution of HBV viremia after HCV treatment in this special population. Ten hemodialysis patients with HBV/HCV coinfection with dominant HCV infection (HBV lower than 2000IU/mL) and significant fibrosis were treated with interferon-alpha 3 MU 3×/week for 12 months and could be followed for at least 36 months after HCV treatment. Six cases of HBV reactivation (60%) during follow-up were observed and 5/6 had been successfully treated for HCV. Patients with HBV reactivation received anti-HBV therapy. Our preliminary findings indicate that treatment of hepatitis C in HBV/HCV coinfected hemodialysis patients may favor HBV reactivation. Thus, continued monitoring of HBV viremia must be recommended and prompt anti-HBV therapy should be implemented.
Hepatitis C virus (HCV) coinfection occurs in 7–15% of patients with chronic HBV infection. It is more frequent in intravenous drug users, in endemic areas of HBV, and in hemodialysis patients.1 Although chronic HBV infection in patients on dialysis is usually asymptomatic and indolent, HBsAg positivity is an independent risk factor for renal graft failure after kidney transplantation.2,3 Moreover, most studies show higher mortality in renal transplant recipients infected with HBV, compared with renal transplant recipients without hepatitis B virus (HBV) infection.
In HBV/HCV coinfected patients, HBV replication is usually suppressed by HCV. Nevertheless, in many cases, a dynamic viral interference between HBV and HCV does exist and viral levels of both viruses may vary during follow-up.4 In patients with undetectable HBV-DNA, treatment for hepatitis C may cause reactivation of HBV replication, as has been reported in several patients, reaching 61.8% in the latest study with pegylated-IFN and ribavirin in non-uremic patients.5 To the best of our knowledge, there is no data regarding long-term follow-up after treatment for HCV in hemodialysis patients with HBV/HCV coinfection. Therefore, this study aimed to assess the evolution of HBV viremia after treatment of hepatitis C in hemodialysis patients with HBV/HCV coinfection.
A retrospective analysis of HBV viremia was performed in a cohort of patients on hemodialysis with HBV/HCV coinfection treated for hepatitis C. We included HBV/HCV coinfected patients with dominant HCV infection (HBV lower than 2000IU/mL) that had been treated with interferon-alpha 3 MU 3×/week for 12 months at the Hepatitis Unit of Federal University of São Paulo from January 1999 to December 2010.
At the end of HCV treatment the patients with no significant HBV viremia could be followed for at least three years in order to assess HBV reactivation. Patients with significant HBV viremia at the end of HCV treatment or with early HBV reactivation during HCV treatment were excluded from further follow-up.
Serum markers HBsAg, HBeAg and anti-HCV were assayed using commercially available enzyme immunoassay kits (Abbott Laboratories, USA).
HBV viral load were performed every three months during follow-up. Serum HCV-RNA was determined at the end of treatment and six months after stopping therapy to evaluate sustained virologic response (SVR) to HCV therapy and determined annually thereafter.
Serum HBV-DNA was quantified by real-time PCR with a lower detection limit of 50IU/mL and HBV genotypes were determined using a line probe assay (INNo-LiPA HBV Genotyping, Innogenetics NV, Belgium). Serum HCV-RNA was detected with an HCV RNA qualitative assay (Cobas Amplicor 2.0, Roche Diagnostics, Basel, Switzerland) with a lower detection limit of 50IU/mL. HCV Genotyping was performed using a line probe assay (INNo-LiPA HCV II, Innogenetics NV, Belgium).
All patients gave a written informed consent prior to liver biopsy and/or IFN treatment. This study protocol was submitted to local Ethics Committees and followed all of the ethical premises of the Helsinki Declaration.
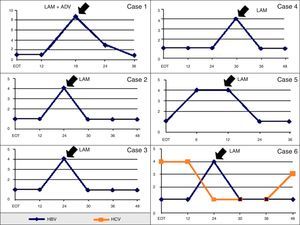
Herein we report long-term follow-up data of 10 HBV/HCV coinfected patients on hemodialysis that could be followed for at least three years after HCV therapy. Baseline clinical and laboratorial characteristics are shown in Table 1. During the follow-up period, 6/10 patients showed HBV reactivation (60%) 22±9 months after the end of HCV treatment and only one case of early reactivation (within the first six months of follow-up) was observed (Table 2). Characteristics of HBV viremia over time in the six cases of HBV reactivation are shown in Fig. 1. In the only case of HBV reactivation without SVR for HCV therapy, a dynamic viral interaction was observed during follow-up (case #6). At first, there was active HCV infection and HBV-DNA was undetectable at the end on HCV treatment; 24 months later, occurred reactivation of HBV infection and HCV-RNA became negative. After introduction of lamivudine for HBV treatment there was a sustained suppression of HBV viral load and reappearance of HCV-RNA.
Demographic, clinical and laboratory characteristics of patients at baseline and during follow-up.
| Total (n=10) | HBV reactivation (n=6) | No HBV reactivation (n=4) | |
|---|---|---|---|
| Age, years, mean±SD | 40±10 | 44±9 | 37±9 |
| Mean time of follow-up, months | 40 | 43 | 38 |
| Mean time of HBV reactivation, months, mean±SD | – | 22±9 | – |
| Male gender, n/% | 6/60% | 4/67% | 2/50% |
| Advanced fibrosis (F≥3) | 2/20% | 0 | 2/50% |
| Anti-HBe (+) at HCV EOT, n/% | 8/80% | 5/83% | 3/75% |
| Loss of HBsAg, n/% | 0 | 0 | 0 |
| Undetectable HBV-DNA at HCV EOT, n/% | 10/100% | 6/100% | 4/100% |
| SVR HCV, n/% | 7/70% | 5/83% | 2/50% |
Abbreviation: SD, standard deviation; SVR, sustained virologic response; EOT, end of treatment.
Virological characteristics before HCV treatment, follow-up at month 6 and most recent follow-up.
| No. | Before HCV treatment | Follow-up month 6 | Most recent follow-up | HBV treatment | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HBeAg status | HBV-DNA (IU/mL) | HBV-GT | HCV-DNA (IU/mL) | HCV-GT | HBeAg Status | HBV-DNA (IU/mL) | HCV-DNA (IU/mL) | Follow-up (months) | HBeAg Status | HBV-DNA (IU/mL) | HCV-DNA (IU/mL) | ||
| 1 | − | 1771 | D | + | 1A | − | Neg. | Neg. | 48 | − | Neg. | Neg. | LAM |
| 2 | − | Neg. | − | 255 | 1A | − | Neg. | + | 48 | − | Neg. | + | LAM |
| 3 | − | 250 | D | + | 1A | − | Neg. | + | 36 | − | Neg. | + | − |
| 4 | − | Neg. | A2 | + | − | − | 2061 | Neg. | 36 | − | Neg. | Neg. | LAM |
| 5 | − | 1409 | D | 134 | − | − | Neg. | Neg. | 40 | − | Neg. | Neg. | LAM+ADV |
| 6 | − | Neg. | − | + | − | − | Neg. | + | 44 | − | Neg. | + | − |
| 7 | − | 1306 | D | 36,672 | 1A | − | 964 | Neg. | 36 | − | Neg. | Neg. | − |
| 8 | − | 167 | D | + | − | − | Neg. | Neg. | 48 | − | Neg. | Neg. | LAM |
| 9 | − | Neg. | − | + | − | − | Neg. | Neg. | 36 | − | Neg. | Neg. | − |
| 10 | − | Neg. | − | + | 1A | − | Neg. | Neg. | 36 | − | Neg. | Neg. | LAM |
Abbreviation: GT, genotype; LAM, lamivudine; ADV, adefovir.
Profiles of serum HCV-RNA and HBV-DNA in six patients with HBV reactivation during follow-up after HCV treatment in months. HCV-RNA and HBV-DNA levels are expressed as log10 IU/mL and lower limit of detection of both tests are 50IU/mL. Abbreviation: EOT, end of hepatitis C treatment; LAM, lamivudine; ADV, adefovir.
No case of HBsAg loss was observed during the follow-up period. In our series, all six cases with significant elevation of HBV viremia were treated promptly regardless of ALT elevation considering that all patients had significant fibrosis at baseline and most of them were already listed for kidney transplantation. In five patients treatment was initiated with lamivudine 10mg/day since at that time entecavir or tenofovir were not available yet. One patient with very high viral load used lamivudine 10mg/day plus adefovir 10mg/week, to reduce the chance of virologic breakthrough. There were no fatal cases due to HBV reactivation and the six patients were released for renal transplantation with undetectable HBV viremia while maintaining continued use of oral antiviral treatment.
This is the first report of HBV reactivation after HCV treatment in coinfected HBV/HCV hemodialysis patients. Our study showed a similar rate of HBV reactivation as recently reported by Yu et al., in a cohort of 76 non-uremic coinfected patients, with pretreatment undetectable HBV-DNA.5 In that study, reappearance of HBV-DNA was found in 47 (61.8%) patients that occurred either during the course of treatment (38.3%) or during post-treatment follow-up (61.7%). Other studies in non-uremic patients also reported a frequent rate of HBV reactivation after HCV treatment, with rates varying from 15.4% to 100%.6–11
In our study, we also observed that HBV reactivation occurred more frequently in patients who had been successfully treated for HCV. Two Asian studies also observed that HBV reactivation rate was significantly higher among those who achieved HCV SVR (44.8 and 33.3%) compared to those without HCV SVR (8.7 and 8.3%).7,11 According to a recent meta-analysis by Liu et al. the risk of reappearance of HBV-DNA in coinfected HBV/HCV patients was three times higher in those who achieved HCV SVR (p=0.009).12
Despite of reappearance of HBV after HCV treatment, it has not resulted in clinically evident hepatitis. Other studies in non-uremic patients also observed the same finding and spontaneous resolution of HBV viremia seems to be frequent.13,14 In the Asian cohort of 76 patients, this phenomenon was transient in 44.7%, intermittent in 25.5% and sustained in 29.8%.5 The impact of the HBV reactivation in the setting of end-stage renal disease may have an unfavorable outcome, considering that these patients have an impaired immume system, affecting both acquired as well as innate immunity.15,16 Thus, reactivation of HBV in this population may lead to greater chance of developing chronic liver disease before renal transplant, and hepatic decompensation after renal transplantation with the use of immunosuppressive therapy.17
Due to the scarcity of data so far, the significance of HBV reappearance after effective treatment of HCV in patients with chronic HBV/HCV coinfection in uremic patients requires further analysis. Up to now, treatment with oral nucleosides/nucleotides in patients under hemodialysis should be started in cases of HBV-DNA >2000IU/mL, regardless of ALT activity especially if they have significant fibrosis on liver biopsy or estimated by a non-invasive marker.18
In conclusion, our data showed that in HBV/HCV coinfected hemodialysis patients reactivation of HBV infection is a frequent phenomenon after treatment of hepatitis C. Continued monitoring of HBV viremia must be recommended for these patients in order to establish early antiviral treatment, especially in patients who are candidates for renal transplantation. Based on this evidence, transplant physicians should be aware of the risk of HBV reactivation in coinfected patients especially when HCV has been eradicated before renal transplantation.
Conflicts of interestThe authors declare no conflicts of interest.