It is believed that Human Papillomavirus (HPV) and Human Immunodeficiency Virus coinfection contributes to increase the risk for cervical intraepithelial injuries. Several factors may contribute to cervical cancer (CC) development, including genetic variants such as TP53 and MDM2 gene polymorphisms.
Materials and methodsA hundred HIV-infected women were examined for HPV detection and its genotypes, as well as the frequencies of the SNPs Arg72Pro and SNP309 and their associations with CC risk factors. Nested Polymerase Chain Reaction (nPCR) was used for HPV detection and PCR-RFLP for TP53 and MDM2 SNP309 genotyping.
ResultsHPV DNA was detected in 68% of samples. A higher frequency of low-risk HPV genotypes (66.7%) was observed when compared to high-risk genotypes (33.3%). Nine different HPV genotypes were identified, with the highest prevalence of HPV-6, followed by HPV-16 and 31. p53 Arg72Arg and SNP309 TG genotype were the most prevalent. HPV genotyping was performed by sequencing.
ConclusionThe data obtained suggest that HIV-infected women are more susceptible to be infected by low-risk HPV (LR-HPV) genotypes than by high-risk (HR-HPV), and Pro72Pro of TP53 gene and TG of MDM2 SNP309 genotypes apparently seem to be protective factors among HIV-infected women for HPV acquisition and HR-HPV infection, respectively, in a sample of Southern Brazilian woman. Future investigations in larger populations are necessary to better understand the potential roles of these SNPs and the behavior of non-oncogenic HPV genotypes in HIV-mediated immunosuppression cases.
It is estimated that cervical cancer (CC) is the second most common type of cancer in the Brazilian female population and the third cause of death in women.1 Today, it is possible to confirm that CC development is closely associated with the Human Papillomavirus (HPV) presence and persistence,2 with HPV DNA detected in up to 99.7% of invasive CC cases across the world.3 The World Health Organization attributes to cervical infection with HPV-16 or -18 about 70% of invasive CC cases in Brazil.4 Cervical intraepithelial neoplasia (CIN) development and cervical cancer in HPV-infected women are associated with risk factors, such as young age, high number of sexual partners throughout life, early sexual debut, smoking, genetics variants, among others.5,6 Moreover, co-infections, such as those with Human Immunodeficiency Virus (HIV) and Chlamydia trachomatis bacterium, may be involved as co-factors to CC development, acting as adjuvants of the neoplastic process.7,8
The polymorphism on codon 72 (Arg72Pro) of TP53 tumor suppressor gene has been extensively investigated regarding association with a wide range of cancers worldwide. In HPV-infected cells, the E6 oncoprotein binds to p53 protein and promotes its degradation through an ubiquitin proteolytic system altering the p53 activity in some processes, such as tumorigenesis, transcription regulation, telomerase activation, and apoptosis, thus resulting in deregulation of the cell cycle.9 Previous studies have shown that the Arg72Arg genotype is related to a higher risk for CC development when compared to the Pro72Pro genotype.10
Studies have shown that a Single Nucleotide Polymorphism (SNP) on promoter region of MDM2 gene, the SNP309 (T to G change on nucleotide 309 of the first intron), results in a higher level of MDM2 mRNA and MDM2 protein, and consequent reduction of the p53 pathway.11 The SNP309 occurs at a relatively high frequency in the general population, and it was shown that it presents a strong association with HPV-mediated cervical carcinogenesis.12
Based on the presented data, this study aimed to investigate the HPV infection spectrum, to identify the most prevalent genotypes, to determine the frequencies of SNPs Arg72Pro and MDM2 SNP309, and their association with possible risk factors for viral persistence and for the development of pre-neoplastic and neoplastic lesions of the uterine cervix in HIV-infected women. The study was conducted in patients of the Service of Specialized Care for HIV/AIDS, College of Medicine, Federal University of Pelotas (Serviço de Assistência Especializada em HIV/SIDA – SAE-UFPel).
Materials and methodsStudy type, characterization and sample sizeThis was a cross-sectional study consisting of 100 HIV-infected women, which were sequentially randomly invited to participate. The eligibility criteria were: not being in the menstrual period, aged between 18 and 45 years, and not being pregnant. Women who had undergone cervical conization and/or hysterectomy were excluded. The Epi-Info 6.0 software was used to calculate the required sample size for the prevalence study, using an estimated HPV prevalence of 80%, with a 95% confidence level and 80% testing power.
LogisticsAll participants completed a standardized questionnaire with the purpose of obtaining information about the patient and other relevant epidemiological and socio-demographic variables. An adapted questionnaire from an intervention study with HIV-infected (HIV+) women conducted at SAE-UFPel was used.13 The Research Ethics Committee of the College of Medicine of the Federal University of Pelotas approved the present study by June 2009, and informed consent was obtained from all participants. All procedures were conducted according to the Helsinki Declaration guidelines.
Sample collection and DNA extractionCytobrushes were collected containing cervical secretion samples from the endocervical region. The collected samples were stored in eppendorf tubes containing 300μL of Cellular Lysis Solution (PUREGENE™Gentra Systems Kit) and routed in an appropriated container to the Laboratory of Functional Genomics (Center for Technology Development – CDTec-UFPel) for viral molecular detection by Polymerase Chain Reaction (PCR). Before DNA extraction, the samples were subjected to enzymatic digestion with 1.5μL of Proteinase K (10mg/mL) and incubated for 16h at room temperature. The extracted DNA was performed according to manufacturer's specifications (PUREGENE™Gentra Systems Kit).
TP53 gene polymorphism analysisThe Arg72Pro polymorphism of TP53 gene was analyzed by PCR using the 72A and 72S primers described by Lin et al.14 The amplification conditions consisted of an initial denaturation at 94°C for 3min followed by 40 cycles at 94°C for 30s, 57°C for 30s, 72°C for 30s, and a final extension at 72°C for 3min.
The PCR product obtained was subjected to enzymatic digestion by RFLP (Restriction Fragment Length Polymorphism) using the Bst UI restriction enzyme for restriction fragments analysis. The fragments obtained were analyzed by agarose gel electrophoresis (2.5% agarose in TBE buffer), stained with GelRed™ (Biotium Inc., CA) and observed under ultraviolet light (UV). The p53 genotypes were characterized according to Lin et al.14
MDM2 SNP309 analysisThe MDM2 T/G SNP309 polymorphism was analyzed by PCR using the primers described by Sotomaa et al.15 The reaction was performed with initial denaturing at 94°C for 10min followed by 30 subsequent cycles at 94°C for 30s, 60°C for 1min, 72°C for 1min, and a final extension at 72°C for 10min. The PCR products were analyzed by agarose gel electrophoresis (2% agarose gel in TBE buffer), stained with GelRed™ (Biotium Inc., CA) and observed under UV. The genotyping was performed by RFLP using the MspA1 l restriction enzyme.15
HPV DNA detection by PCRThe HPV DNA detection was performed by the nested PCR (nPCR) technique, which is performed in two stages. The first stage used a pair of external primers (MY09/11) previously described by Manos et al.,16 and the second stage used a pair of internal primers (GP5/6) described by Snijders et al.17 Both reactions were performed with initial denaturing at 95°C during 9min and final extension at 72°C for 5min.18,19 The reaction conditions for the first stage consisted of 40 subsequent cycles of 94°C during 30s for denaturation, 45°C during 30s for annealing, and 72°C for 30s for extension. For the second stage were used 40 subsequent cycles of 95°C for 1min, 55°C for 1min, and 72°C for 1min for denaturation, annealing and extension, respectively.19 The PCR products obtained were subjected to agarose gel electrophoresis (1.0% and 2.0% agarose for the first and second rounds, respectively) and observed under UV. A reaction positive control (RPC) consisting of 450pb corresponding to HeLa cells (HPV-16) and a negative control (NC) without any DNA were used.
Sequencing of the PCR productsThe HPV DNA-positive samples were sequenced for identification of the present genotypes. The products obtained from nPCR were purified using the Illustra™ GFX™ PCR DNA and Gel Band Purification Kit (GE Health Care) and sequenced on a MegaBACE 500 (GE Healthcare).
Sequence analysesForward and reverse sequences were aligned and a consensus sequence was obtained for each case using VECTOR NTI® 10.0 software. The genotypes were determined comparing the consensus sequence with reference sequences deposited in DNA databases through the nucleotide Basic Local Search Tool (BLASTn). For the samples edition and alignment, the VECTOR NTI® 10.0 software was used.
Data analyzeData was entered by two different data clerks with the software Epi-Info® Windows version in order to make a further comparison and, thus, ensure better data quality. An automatic data checking was performed at the time of typing using the Epi-Info®Check function for consistency and amplitude checking. To identify and correct coding, proofreading and typing inconsistencies, a data cleaning was performed by obtaining frequencies of the collected variables. The analysis was performed using the SPSS® v16.0 software. Chi-square tests (χ2) were used for the univariate analyses. Multivariate logistic regression analysis was also performed. Variables remained at the model if their level of significance was below 0.20, as potential confounders for the next level. A p-value<0.05 was considered statistically significant.
ResultsMost participants were aged between 40 and 45 years (53%), were white (51%), 45.3% had between five and eight years of education, were married or in a stable relationship (64.4%), and the overriding majority had per capita income of one minimum wage (96.6%). Regarding behavioral variables, 59.6% were non-smokers, 60% reported not having consumed alcohol in the last four weeks, 63.3% reported having used a condom at last sexual intercourse, 56% were aged between 12 and 15 years at sexual debut, 40.8% had more than nine sexual partners throughout life, and over 90% reported never had exchanged sex for money and/or drugs.
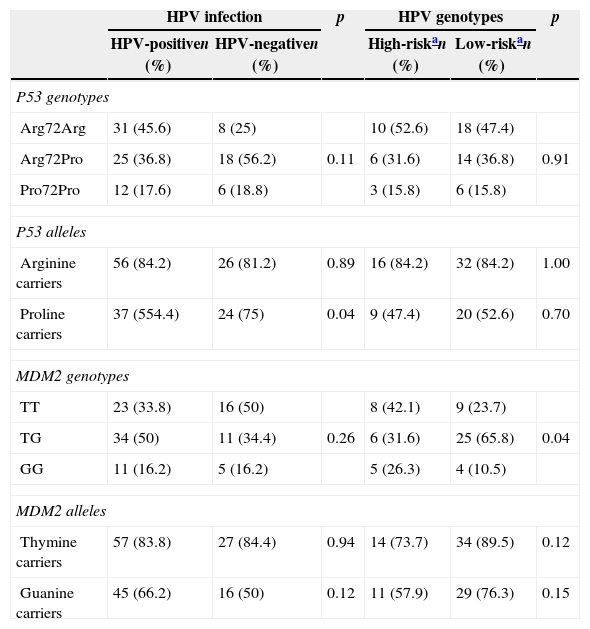
The TP53 genotyping showed that, out of the 100 samples analyzed, 43% presented Arg72Pro genotype, 39% were Arg72Arg and 18% were Pro72Pro. The alleles frequency showed 82% for the A (AA+AP) allele and 61% for the P (AP+PP) allele. The MDM2 SNP309 genotyping showed a higher frequency for the TG genotype (45%). Moreover, the T (TT+TG) allele was the most frequent (84%) compared to the G (GG+TG) allele (61%).
HPV infection was observed in 68% of the samples. Nine different HPV genotypes were found, with a higher prevalence of HPV-6, followed by HPV-16, HPV-31, HPV-11, HPV-18, HPV-35, HPV-45, HPV-56 and HPV-81. Eleven samples could not be genotyped by sequencing, and these were categorized as HPV-X. According to the epidemiologic classification of HPV types associated with cervical cancer, 33.3% of the samples were high-risk oncogenic HPV (HR-HPV) and 66.7% were low-risk oncogenic HPV (LR-HPV).20
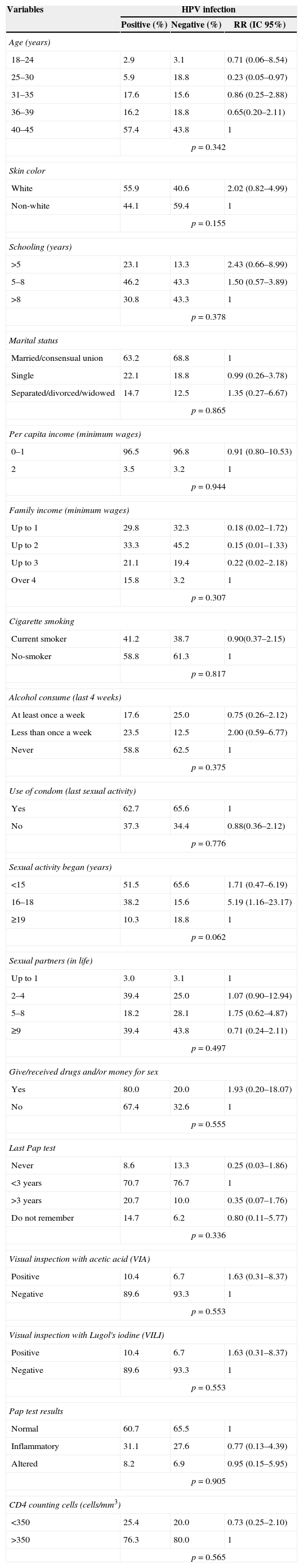
Table 1 shows the univariate analysis, having HPV infection as outcome related to socioeconomic, demographic and behavioral variables. There was no statistically significant association with any of the variables. Table 2 shows the frequency distribution of genotypes and alleles of Arg72Pro and MDM2 SNP309. The Arg72Arg genotype of the TP53 gene was the most prevalent among the HPV+ group (45.6%), predominating A (AA+AP) allele (82.4%) compared to P (AP+PP) allele (54.4%). The MDM2 SNP309 presented the TG genotype as the most prevalent (50%), as well as the T (TT+TG) allele (83.8%) when compared to the G (GG+TG) allele (66.2%). The LR-HPV infected group showed frequencies similar to those shown by the HR-HPV infected group, except for some variables. Regarding the TP53 gene polymorphism, it was observed that 52.6% of the HR-HPV infected samples and 47.4% of the LR-HPV infected samples presented the Arg72Arg genotype. In addition, the A (AA+AP) allele was more frequent in both infections (LR and HR) when compared to the P (AP+PP) allele (p<0.05). Furthermore, the MDM2 SNP309 genotyping showed a higher frequency of TT genotype (42.1%) in the HR-HPV group, as well as higher frequency of TG genotype (65.8%) in the LR-HPV group (p<0.05). The T (TT+TG) allele was more frequent when compared to the G (GG+TG) allele in both groups.
Interaction of HPV infection and the oncogenic potential risk on the variables of HIV-positive women.
| Variables | HPV infection | ||
|---|---|---|---|
| Positive (%) | Negative (%) | RR (IC 95%) | |
| Age (years) | |||
| 18–24 | 2.9 | 3.1 | 0.71 (0.06–8.54) |
| 25–30 | 5.9 | 18.8 | 0.23 (0.05–0.97) |
| 31–35 | 17.6 | 15.6 | 0.86 (0.25–2.88) |
| 36–39 | 16.2 | 18.8 | 0.65(0.20–2.11) |
| 40–45 | 57.4 | 43.8 | 1 |
| p=0.342 | |||
| Skin color | |||
| White | 55.9 | 40.6 | 2.02 (0.82–4.99) |
| Non-white | 44.1 | 59.4 | 1 |
| p=0.155 | |||
| Schooling (years) | |||
| >5 | 23.1 | 13.3 | 2.43 (0.66–8.99) |
| 5–8 | 46.2 | 43.3 | 1.50 (0.57–3.89) |
| >8 | 30.8 | 43.3 | 1 |
| p=0.378 | |||
| Marital status | |||
| Married/consensual union | 63.2 | 68.8 | 1 |
| Single | 22.1 | 18.8 | 0.99 (0.26–3.78) |
| Separated/divorced/widowed | 14.7 | 12.5 | 1.35 (0.27–6.67) |
| p=0.865 | |||
| Per capita income (minimum wages) | |||
| 0–1 | 96.5 | 96.8 | 0.91 (0.80–10.53) |
| 2 | 3.5 | 3.2 | 1 |
| p=0.944 | |||
| Family income (minimum wages) | |||
| Up to 1 | 29.8 | 32.3 | 0.18 (0.02–1.72) |
| Up to 2 | 33.3 | 45.2 | 0.15 (0.01–1.33) |
| Up to 3 | 21.1 | 19.4 | 0.22 (0.02–2.18) |
| Over 4 | 15.8 | 3.2 | 1 |
| p=0.307 | |||
| Cigarette smoking | |||
| Current smoker | 41.2 | 38.7 | 0.90(0.37–2.15) |
| No-smoker | 58.8 | 61.3 | 1 |
| p=0.817 | |||
| Alcohol consume (last 4 weeks) | |||
| At least once a week | 17.6 | 25.0 | 0.75 (0.26–2.12) |
| Less than once a week | 23.5 | 12.5 | 2.00 (0.59–6.77) |
| Never | 58.8 | 62.5 | 1 |
| p=0.375 | |||
| Use of condom (last sexual activity) | |||
| Yes | 62.7 | 65.6 | 1 |
| No | 37.3 | 34.4 | 0.88(0.36–2.12) |
| p=0.776 | |||
| Sexual activity began (years) | |||
| <15 | 51.5 | 65.6 | 1.71 (0.47–6.19) |
| 16–18 | 38.2 | 15.6 | 5.19 (1.16–23.17) |
| ≥19 | 10.3 | 18.8 | 1 |
| p=0.062 | |||
| Sexual partners (in life) | |||
| Up to 1 | 3.0 | 3.1 | 1 |
| 2–4 | 39.4 | 25.0 | 1.07 (0.90–12.94) |
| 5–8 | 18.2 | 28.1 | 1.75 (0.62–4.87) |
| ≥9 | 39.4 | 43.8 | 0.71 (0.24–2.11) |
| p=0.497 | |||
| Give/received drugs and/or money for sex | |||
| Yes | 80.0 | 20.0 | 1.93 (0.20–18.07) |
| No | 67.4 | 32.6 | 1 |
| p=0.555 | |||
| Last Pap test | |||
| Never | 8.6 | 13.3 | 0.25 (0.03–1.86) |
| <3 years | 70.7 | 76.7 | 1 |
| >3 years | 20.7 | 10.0 | 0.35 (0.07–1.76) |
| Do not remember | 14.7 | 6.2 | 0.80 (0.11–5.77) |
| p=0.336 | |||
| Visual inspection with acetic acid (VIA) | |||
| Positive | 10.4 | 6.7 | 1.63 (0.31–8.37) |
| Negative | 89.6 | 93.3 | 1 |
| p=0.553 | |||
| Visual inspection with Lugol's iodine (VILI) | |||
| Positive | 10.4 | 6.7 | 1.63 (0.31–8.37) |
| Negative | 89.6 | 93.3 | 1 |
| p=0.553 | |||
| Pap test results | |||
| Normal | 60.7 | 65.5 | 1 |
| Inflammatory | 31.1 | 27.6 | 0.77 (0.13–4.39) |
| Altered | 8.2 | 6.9 | 0.95 (0.15–5.95) |
| p=0.905 | |||
| CD4 counting cells (cells/mm3) | |||
| <350 | 25.4 | 20.0 | 0.73 (0.25–2.10) |
| >350 | 76.3 | 80.0 | 1 |
| p=0.565 | |||
Interaction of Arg72Pro and MDM2 SNP309 on the HPV infection and oncogenic risk status.
| HPV infection | p | HPV genotypes | p | |||
|---|---|---|---|---|---|---|
| HPV-positiven (%) | HPV-negativen (%) | High-riskan (%) | Low-riskan (%) | |||
| P53 genotypes | ||||||
| Arg72Arg | 31 (45.6) | 8 (25) | 10 (52.6) | 18 (47.4) | ||
| Arg72Pro | 25 (36.8) | 18 (56.2) | 0.11 | 6 (31.6) | 14 (36.8) | 0.91 |
| Pro72Pro | 12 (17.6) | 6 (18.8) | 3 (15.8) | 6 (15.8) | ||
| P53 alleles | ||||||
| Arginine carriers | 56 (84.2) | 26 (81.2) | 0.89 | 16 (84.2) | 32 (84.2) | 1.00 |
| Proline carriers | 37 (554.4) | 24 (75) | 0.04 | 9 (47.4) | 20 (52.6) | 0.70 |
| MDM2 genotypes | ||||||
| TT | 23 (33.8) | 16 (50) | 8 (42.1) | 9 (23.7) | ||
| TG | 34 (50) | 11 (34.4) | 0.26 | 6 (31.6) | 25 (65.8) | 0.04 |
| GG | 11 (16.2) | 5 (16.2) | 5 (26.3) | 4 (10.5) | ||
| MDM2 alleles | ||||||
| Thymine carriers | 57 (83.8) | 27 (84.4) | 0.94 | 14 (73.7) | 34 (89.5) | 0.12 |
| Guanine carriers | 45 (66.2) | 16 (50) | 0.12 | 11 (57.9) | 29 (76.3) | 0.15 |
The multivariate analyses showed no statistically significant differences and their results are not reported here.
DiscussionStudies have shown that HPV infection is most common in HIV-infected women and it is believed that HIV contributes to increase HPV viral load and hence its persistence, increasing the risk for cervical intraepithelial lesions.8 The HPV infection prevalence in this study was 68%. These observations are consistent with a meta-analysis study reported by Clifford et al., who observed higher HPV prevalence in HIV-infected women in Brazil and Mexico (57.3%) compared to the global prevalence (36.5%).21
In this study women older than 40 years, with low income, early sexual debut and high number of sexual partners throughout life showed greater risk to acquire HPV infection. Sauvaget et al. in a study conducted with women with average age of 39 years from rural areas of India, found an association with low income, early age at first pregnancy, and low educational level.22 They suggested that constant increase in HPV prevalence in middle-aged women may result from reduced ability of the host to eliminate HPV infection, nutritional deficiencies, or from the virus ability to become latent and be reactivated due to a failure of immune surveillance or hormonal factors associated to older age. Moreover, the HR-HPV prevalence usually shows a constant decline with the increasing age seen in high-income countries, in contrast to the U-shaped curves reported in some Latin-American countries. Here, an increased prevalence of HPV infection in the age group of 35–44 years old has been reported in a cross-sectional study performed in eleven countries with different cervical cancer incidences.23
The genotyping by sequencing showed the presence of nine different HPV genotypes, with a higher prevalence for HPV-6, followed by HPV-16 and -31 in this study. Although most studies show that HPV-16 genotype is the most prevalent worldwide,21 this data is in line the report by Corrêa et al., who observed a prevalence of 63.9% of HPV-6 genotype followed by HPV-16 genotype as the second most prevalent (48.5%).24 Levi et al. observed similar data, with higher prevalence of HPV-6 genotype in HIV-infected women (87%).25 McKenzie et al., in a meta-analysis study, observed that different geographic areas, such as Zambia, Brazil and Rochester (New York, United States), show different HR-HPV infections by less prevalent genotypes when compared to the population in general.26 Furthermore, they show that cervical malignancy has a different behavior in HIV-infected patients, as well as benign manifestations of HPV.
Apparently, the HIV infection exerts a multifactorial oncogenic function in cervical cancer development, interfering in the immune function and also in the direct promotion of the lesion development. The HIV-1 Tat protein seems to promote the cellular proliferation and positively regulate the E6 and E7 HPV genes, responsible for the malignant cellular transformation.27,28 Recent data indicate that significant changes occur in the uterine cervix in HPV/HIV co-infected women suggesting that the immune response appears to be negatively regulated, except for the RANTES protein (Regulated on Activation, Normal T cell Expressed and Secreted), which has been found to be increased.29 RANTES levels were found significantly increased in plasma and tissue from patients with breast cancer and cervical cancer, suggesting that positive regulation of RANTES and other changes in cervical tissue associated with HPV/HIV co-infection (such as those mediated by HIV-1 Tat protein) would permit less aggressive HPV genotypes to cause malignant transformation.30 Some authors suggest that this variation of genotypes is due to socioeconomic and behavioral characteristics, which may be different in HIV-infected women, besides the geographic region where the research is conducted.31 With regard to HPV infection stratified by genotype, further studies comparing innate immunity between HIV+ and HIV− women are necessary. These studies will help to individualize treatment and prevention in the group of women at increased risk of morbidity and mortality due to HPV-related diseases.
The ability of the persistent HPV infection to cause malignant transformation is associated to the viral DNA integration into the genome of the host.32 However, some studies have challenged this statement. These studies indicate that the necessity of integration for the carcinogenesis varies with the HPV genotype (for example, only 50% of the HPV-16 genome integrates with the host, while HPV-18 needs to integrate 94%).33 This distinction is particularly important for the comprehension of the disease etiology in HIV-infected patients, since these are commonly infected by HPV genotypes that require integration with the host genome for the carcinogenesis development. Furthermore, recent studies have described the association between the integration rate and the cervical dysplasia severity.34
Concerning the Arg72Pro TP53 gene polymorphism, the present study found no statistically significant relation between observed genotypes, frequency of the Arg and Pro alleles and HPV presence. Moreover, it is worth noting that the controversial results regarding the association between TP53 gene polymorphism and cervical cancer can occur due to some factors, such as differences in the TP53 polymorphism among ethnical groups, sample size, choice of the control to be used and DNA obtaining source.35 For an example, a study using normal cervical samples and high- and low-risk lesion samples failed to show an association between the presence of the polymorphism and frequency of the Arg and Pro alleles with increased risk for CC,12 suggesting that the association between the TP53 gene polymorphism and the development of HPV-associated cervical neoplasia is improbable. However, in a meta-analysis study performed one year later, it was observed significantly higher odds of progression from SIL to CC with the p53 Arg allele [OR 1.37; 95% CI, 1.15–1.62; p<0.001] in HPV-positive individuals.36
There are a few studies correlating MDM2 SNP309 to CC susceptibility, although SNP309 presents a relatively high frequency in the general population and its presence has been associated with the acceleration of tumorigenesis and time of tumor onset.10 In this study, TT and TG genotypes were significantly more frequent in HR-HPV and LR-HPV groups, respectively, but presence or frequency of the T and G alleles was not significantly associated with HPV infection. There is an inconsistency regarding the role of MDM2 SNP309 in susceptibility to the CC development. Nunobiki et al. found a prevalence of 78.8% for the TG genotype among high-grade squamous intraepithelial lesion (HSIL) samples when compared with low-grade squamous intraepithelial lesion (LSIL) samples and controls, among HR-HPV positive samples and, specially, the HPV-16 and -18 genotypes.11 However, there was no association between the samples and the frequencies of T and G alleles. Meissner et al. in a study conducted in Northeastern Brazil for identification of the MDM2 SNP309 in 72 CC samples and 100 normal samples by PCR found no statistically significant association between the SNP309 and the risk and early diagnosis for CC.37 Also, no difference was observed for the frequency of alleles or genotypes among the group of patients diagnosed with CC at an early age (below 40 years) and the group of older patients. On the other hand, Arvantis and Spandidos through the analysis of mRNA expression profiles of 24 G1- and S-phase checkpoint genes in 35 cervical carcinomas samples, 26 HSIL samples, 33 LSIL samples, and 28 normal cervical samples (used as controls), showed that the MDM2 was found positively regulated in SIL, suggesting that MDM2 gene is a potential candidate for cervical neoplasia development.38 Although this data suggest that HR-HPV infection and the MDM2 SNP309 may be associated with cervical carcinogenesis, in our study there were no samples from patients with CC, which can be considered a limiting factor for these two associations types, since the polymorphisms of both MDM2 and TP53 genes are associated with increased risk for CC and not with susceptibility to HPV infection.
A better understanding of the HPV infection evolution in these women, the viral persistence occurrence, and possible factors associated to the malignant cervical lesions development are necessary for establishing more appropriate CC screening routines in this population. This is essential for the proposition of more appropriate monitoring strategies and treatment according to the Brazilian health service reality, as well as patients.
ConclusionCurrent recommendations are partly based on the knowledge of professionals about CC management and HIV infection in women. The data obtained in this study suggest that HIV-infected women are more susceptible to be infected by the LR-HPV genotypes than by the high-risk HR-HPV. Additionally, Pro72Pro of TP53 gene and TG of MDM2 SNP309 genotypes apparently seem to be protective factors among HI-infected women for HPV acquisition and HR-HPV infection, respectively, in a sample of Southern Brazilian woman. Moreover, further studies are necessary for the investigation of the behavior of low-risk genotypes infecting HIV-mediated immunosuppressed cases.
Conflicts of interestThe authors declare no conflicts of interest.
The authors are grateful to Brazilian National Research Council (CNPq).