Recently, many studies have evaluated HPV vaccine safety and adverse effects. Two vaccines have been recently evaluated in randomized controlled trials: the bivalent vaccine for HPV 16 and 18 (Cervarix, GlaxoSmithKline Biologicals, Rixensart, Belgium) and the quadrivalent vaccine for HPV 6, 11, 16, and 18 (Gardasil, Merck and Co., Inc., Whitehouse Station, NJ). We have performed a systematic review of all randomized controlled trials in which HPV vaccines were compared with placebo regarding safety, tolerability and adverse effects. Studies were searched up to March 2013 in the databases: Pubmed, Embase, Scielo and Cancerlit. Odds Ratios (OR) of most incident adverse effects were obtained. Twelve reports, involving 29,540 subjects, were included. In the HPV 16/18 group, the most frequently reported events related to the vaccine were pain (OR 3.29; 95% CI: 3.00–3.60), swelling (OR 3.14; 95% CI: 2.79–3.53) and redness (OR 2.41; 95% CI: 2.17–2.68). For the HPV 6/11/16/18 group the events were pain (OR 2.88; 95% CI: 2.42–3.43) and swelling (OR 2.65; 95% CI: 2.0–3.44). Concerning the HPV 16/18 vaccine, pain was the most common outcome detected. These effects can be due to a possible VLP-related inflammation process. Fatigue was the most relevant general effect observed followed by fever, gastrointestinal symptoms, and headache. In the HPV 6/11/16/18 group, only general symptoms, pain and swelling were observed. Pain and swelling were the most frequent. Comparing HPV 16/18 to HPV 6/11/16/18 vaccines, the former presented more adverse effects, perhaps because there are many more trials evaluating the bivalent vaccine. Other studies are needed to clarify this issue.
Cervical cancer is the third most common cancer in women and the fourth most common cause of death worldwide.1 Infection with certain types of human papillomavirus (HPV) is necessary to develop cervical cancer.2–4 This has led to an increase in effectiveness of screening for cervical cancer using Pap smears and the development of primary prevention through the use of prophylactic vaccines against HPV.5–11
The prophylactic vaccine stimulates the development of the humoral immune response, which occurs after contact with the “virus-like particles” (VLPs), which are non-infectious structures and simulate a natural HPV infection. The two oncogenic types included in both vaccines are HPV 16 and 18, responsible for at least 70% of the cases of cervical cancer worldwide. In the case of the quadrivalent vaccine, it also included two non-oncogenic types of HPV, 6 and 11, responsible for approximately 90% of cases of anogenital condylomata acuminata.12
Safety and tolerability of both vaccines have been evaluated extensively with similar profiles in the vaccinated and control groups, irrespective of age or ethnicity.1 Studies about safety assessment indicated that local and systemic injection-related symptoms were generally mild. Serious adverse effects (AE) that are considered to be vaccine related are rare and similar to other vaccine types.13,14
Studies indicate that the most common AE is injection-related local reaction, such as pain, swelling and erythema with a rate of 95% of light to moderate intensity.15,16 Regarding systemic symptoms, fever, nausea, vomiting, dizziness, myalgia and diarrhea were reported.15,17,18 Severe AE, such as severe headache with hypertension, gastroenteritis and bronchospasm, were described in 0.5%.15 There are more data available of AE associated with the quadrivalent vaccine than the bivalent vaccine; however, the major AE for the latter vaccine is also in the injection-related local pain (78%).15
Both HPV vaccines are classified as Pregnancy Category B by the FDA. Therefore, the vaccine is not recommended for pregnant women, because there are not enough data to ensure safety to the fetus.19,20
Studies have also demonstrated efficacy and safety of the vaccine in heterosexual and homosexual men.21 This is important as HPV also causes disease in men.
The safety profiles of HPV vaccines have been confirmed by their huge use worldwide, and they has been included in immunization schedules of 28 countries. So far, there has not been any absolute contraindication for the use of these vaccines.15 The vaccines are well tolerated and the number of systemic AE, serious AE, and discontinuations due to a serious event are similar between the two vaccines and control groups.19
The purpose of this study was to evaluate safety and AE of HPV vaccines.
Materials and methodsThis study adhered to PRISMA guidelines.22 As a secondary study, no Institutional Review Board approval was required.
Inclusion criteriaStudies meeting the following criteria were included: (1) double-blind randomized clinical trials evaluating safety and adverse effects of human papillomavirus (HPV) vaccines (against 16/18 and/or 6/11/16/18 serotypes); (2) studied subjects were older than nine years old; (3) exclusion of study participants with high risk of contracting, such as female sex workers and women who were sexual partners of HIV-infected men, and (4) exclusion of pregnant women.
Search and selection of literatureThe studies were identified by a wide literature search of databases (PubMed, Embase, Scielo and Cancerlit) following medical subject heading terms and/or text words: (vaccines OR vaccination) AND (randomized controlled trial) OR (controlled clinical trial) OR (randomized controlled trials) OR (random location) OR (double blind method) OR (single blind method) OR (clinical trial) AND (Human papillomavirus) OR (HPV) OR (papilloma virus) OR (papillomavir*). Reference lists of the identified publications for additional pertinent studies were reviewed. No language restrictions were imposed. Three researchers (AGM, HMR and RNC) searched for articles published up to March 2013.
Study identification and selection is illustrated in the flow diagram in Fig. 1. After searching the databases, 2494 potentially relevant papers were identified, of which 2426 were excluded: 2267 after reviewing the title, and 159 after reviewing the abstract. Reviews were done by AGM, HMR, and RNC; disagreements were solved by a fourth reviewer (AKG). Thus, 68 papers met the criteria and were reviewed in full. There were no articles in languages other than English, which, based on the abstract review, met the inclusion criteria. After full review, 46 papers were not considered to have adequate methodological quality according to the Jadad Scale.23 Finally, nine repeated studies were found (they were present in two databases at the same time), and two studies that used the same group, showing the same results (in this case, only the first publication was included). Finally, 12 papers were approved for data extraction (Fig. 1).
Data extractionVarious study characteristics were extracted from the original research and included in the meta-analysis. The data included the first authors’ last names, year of publication, place of the study, follow-up period, inclusion/exclusion criteria, type of vaccine used, type of control used, and age interval (Table 1).
Study design features of safety of HPV vaccines.
| Author, year | Local | Follow-up | Inclusion criteria | Vaccine type | Control | Age interval | n |
|---|---|---|---|---|---|---|---|
| Harper, 2004 | Multicenter | 27 months | No more than six sexual partners; no history of an abnormal Pap test or ablative or excisional treatment of the cervix; no ongoing treatment for external condylomata; cytologically negative, seronegative for HPV-16 and HPV-18 antibodies by ELISA, and HPV-DNA-negative by PCR for 14 high-risk HPV types no more than 90 days before study entry. | 16/18 | Aluminum hydroxide. | 15–25 | 1113 |
| Bhatla, 2010 | India | 7 months | Nonpregnant or planning to become pregnant; not taking any other investigational products or steroids. | 16/18 | Aluminum hydroxide. | 18–35 | 354 |
| Kim, 2010 | Korea | 7 months | Nonpregnant or planning to become pregnant; not taking any other investigational products or immune-modifying drugs and not breastfeeding during the study. | 16/18 | Aluminum hydroxide. | 10–14 | 321 |
| Medina, 2010 | Multicenter | 14 months | Healthy girls. | 16/18 | Hepatitis A virus vaccine | 12+- | 2067 |
| Ngan, 2010 | Hong Kong | 7 months | Healthy women aged 18–35 years. | 16/18 | Aluminum hydroxide. | 18–35 | 294 |
| Kim, 2011 | Korea | 7 months | Nonpregnant and agreed to use adequate contraceptive precautions over the vaccination period. | 16/18 | Aluminum hydroxide. | 15–25 | 225 |
| Szarewski, 2011 | Multicenter | 4 years | Nonpregnant; no more than six lifetime sexual partners before enrolment; agreed to use adequate contraception over the vaccination period and had an intact cervix. | 16/18 | Hepatitis A virus vaccine. | 15–25 | 18,644 |
| Khatun, 2012 | Bangladesh | 16 months | Unmarried and sexually unexposed. | 16/18 | No vaccine. | 9–13 | 67 |
| Reisinger, 2007 | Multicenter | 18 months | Nonpregnant; must not have had a febrile illness (fever more than 37.8°C [100°F]) at vaccination. | 6/11/16/18 | Aluminum hydroxide. | 9–15 | 1781 |
| Kang, 2008 | Korea | 7 months | Nonpregnant; must not have had a febrile illness (fever more than 37.8°C [100°F]) at vaccination; subjects aged 9–15 years: no sexual experience before and no plan to have sexual experience during the study period; subjects aged 16–23: less than four male and/or female sexual partners at enrollment and were required to use effective contraception during the study period. Exclusion criteria: enrollment in studies of other investigational agents; any HPV vaccination, history of allergy to vaccine compound; thrombocytopenia; history of vaccination within 14 days from enrollment; receipt of blood or blood-derived products within the 6 months preceding injection, and immunosuppression. Subjects aged 16–23 years of age: have not had a prior Pap test showing a squamous intraepithelial lesion or worse and/or a biopsy indicating CIN or worse. | 6/11/16/18 | Same adjuvant. | 9–23 | 176 |
| Lazcano-Ponce, 2009 | Mexico | 48 months | Nonpregnant; four or less sexual partners during their lifetime. | 6/11/16/18 | Aluminum hydroxide. | 18–23 | 679 |
| Muñoz, 2009 | Multicenter | 48 months | Nonpregnant; no history of genital warts or present or past; not immunocompromised (HIV or other), not undergone hysterectomy; required to use effective contraception until month 7 of the study. Women with any previous cervical surgical procedure and those having undergone a cervical biopsy within the past 5 years were excluded. | 6/11/16/18 | Aluminum hydroxide. | 24–45 | 3819 |
Data were entered in the Review Manager software (RevMan 5.2),24 which allows the user to enter protocols as well as complete reviews, including text, features of studies, comparison tables, and study data, as well as to perform meta-analysis of the entered data. Odds Ratios were obtained.
ResultsTwelve reports of HPV vaccine safety and AE involving 29,540 subjects were included. The design features of the clinical trials to assess safety of the chosen HPV vaccines are indicated in Table 1. Eight studies17,18,25–30 evaluated HPV 16/18 vaccines, including 23,085 subjects; two studies18,30 approved for systematic review were not included in the meta-analysis as they did not compare data from experimental and control groups. Four studies16,31–33 including 6455 subjects evaluated HPV 6/11/16/18 vaccine (Table 1).
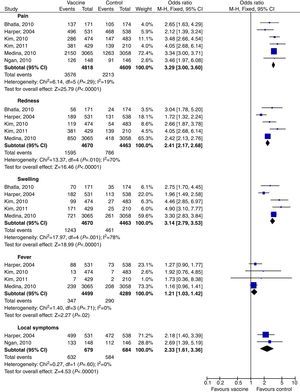
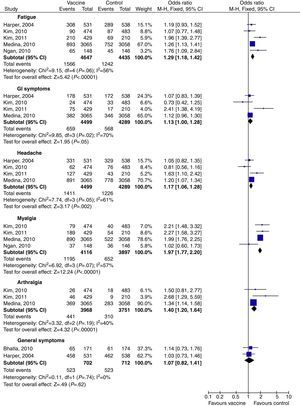
Tables 2 and 3 show a summary of the main AE. The most common AE seen with HPV 16/18 vaccine were, in this sequence: pain, fatigue, redness, swelling, fever, GI symptoms (diarrhea, nausea, vomiting), headache, myalgia and arthralgia. The global Odds Ratios (M-H, Fixed, 95% CI – calculated after meta-analysis) compared to their respective control groups are shown in Table 2. Three studies17,25,27 separated their results as general symptoms and injection-site symptoms; each was analyzed separately. The injection-site events most related to the bivalent vaccine were pain (OR 3.29; 95% CI: 3.00–3.60), swelling (OR 3.14; 95% CI: 2.79–3.53), and redness (OR 2.41; 95% CI: 2.17–2.68). GI symptoms and general symptoms did not appear to be vaccine related (OR 1.13; 95% CI: 1.00–1.28 and OR 1.07; 95% CI: 0.82–1.41 respectively) (Table 2 and Figs. 2 and 3).
Meta-analysis of HPV side effects.
| Outcome | Studies | Doses | Vaccine type | Effect estimate |
|---|---|---|---|---|
| Pain | 6 | 9427 | 16/18 | 3.29 [3.00, 3.60] |
| Fatigue | 5 | 9082 | 16/18 | 1.29 [1.18, 1.42] |
| Redness | 5 | 9133 | 16/18 | 2.41 [2.17, 2.68] |
| Swelling | 5 | 9133 | 16/18 | 3.14 [2.79, 3.53] |
| Fever | 4 | 8788 | 16/18 | 1.21 [1.03, 1.42] |
| GI symptoms | 4 | 8788 | 16/18 | 1.13 [1.00, 1.28] |
| Headache | 4 | 8788 | 16/18 | 1.17 [1.06, 1.28] |
| Myalgia | 4 | 8013 | 16/18 | 1.97 [1.77, 2.20] |
| Arthralgia | 3 | 7719 | 16/18 | 1.40 [1.20, 1.64] |
| Local symptoms | 2 | 1363 | 16/18 | 2.33 [1.61, 3.36] |
| General symptoms | 2 | 1414 | 16/18 | 1.07 [0.82, 1.41] |
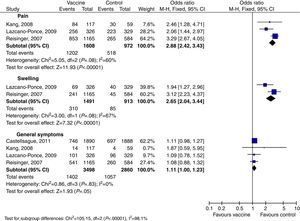
| Pain | 3 | 2580 | 6/11/16/18 | 2.88 [2.42, 3.43] |
| Swelling | 2 | 2404 | 6/11/16/18 | 2.65 [2.04, 3.44] |
| General symptoms | 4 | 6358 | 6/11/16/18 | 1.11 [1.00, 1.23] |
Statistical method was Odds Ratio (M-H, Fixed, 95% CI).
Meta-analysis of adverse events associated with HPV 6/11/16/48 and HPV 16/18 vaccines.
| Outcome | n HPV 6/11/16/18 (experimental group) | n HPV 16/18 (control group) | Total | Effect estimate |
|---|---|---|---|---|
| Pain | 1608 | 4818 | 6426 | 0.97 [0.85, 1.11] |
| Swelling | 1491 | 4670 | 6161 | 1.38 [1.20, 1.59] |
| Fever | 579 | 4499 | 5078 | 1.19 [0.84, 1.68] |
| Local symptoms | 1889 | 679 | 2568 | 4.08 [2.98, 5.59] |
| General symptoms | 3498 | 702 | 4200 | 4.37 [3.64, 5.24] |
Statistical method was Odds Ratio (M-H, Fixed, 95% CI). HPV 6/11/16/18 vaccine was considered the control group and HPV 16/18 the experimental group.
With the HPV 6/11/16/18 vaccine, fewer AE were reported because only four papers were ultimately approved. AE were general symptoms, pain, and swelling (Table 2). Again, general symptoms did not appear to be vaccine related. Pain and swelling, however, were vaccine related: OR 2.88; 95% CI: 2.42–3.43 and OR 2.65; 95% CI: 2.0–3.44, respectively (Table 2 and Fig. 4).
It was possible to perform a meta-analysis comparing HPV 6/11/16/18 and HPV 16/18 vaccines. The statistical method was again Odds Ratio (M-H, Fixed, 95% CI) considering HPV 6/11/16/18 vaccine as control group and HPV 16/18 as experimental group. Thus, HPV 6/11/16/18 vaccine appears to be less associated with swelling, fever, and general symptoms than the HPV 16/18 (Table 3). Fever was not associated to any of the vaccines.
DiscussionAdministration of HPV vaccine has been well tolerated in different groups. The proportion of subjects reporting AE or discontinuing due to an AE is low and similar for the two vaccines.
Occurrence of AE was reported in all randomized control trials.16–18,25–33 As anticipated, the most commonly reported AE were injection-site reactions16–18,25–33; most of these AE were mild or moderate in intensity. These AE were more common among those who received HPV vaccines when compared with placebo subjects. Headache and fatigue were the most common vaccine-related systemic AE seen in approximately 50–60% of all participants.25–30 On the other hand, most of these AE were mild or moderate in intensity.16–18,25–33 Similarly to another study,34 serious vaccine related AE were not significantly different, and there were no registered vaccine related deaths in all studies.16–18,25–34
On the other hand, a very recent systematic review also reported barriers to preventive human papillomavirus vaccination among adolescent girls and young women: 21 barriers to vaccination were identified. Cost was the most frequently reported barrier, followed by beliefs that vaccination was unnecessary, and concerns regarding vaccine safety and side effects.35
Recently an interesting cohort study conducted in Denmark and Sweden identified no safety signals with respect to autoimmune, neurological and venous thromboembolic adverse events after immunization of adolescent girls with quadrivalent human papillomavirus vaccine. In this multicenter study, 997,585 girls aged 10–17 were approached from whom 296,826 received a total of 696,420 quadrivalent HPV (qHPV) vaccine doses. In this research, incident hospital diagnosed autoimmune, neurological or venous thromboembolic events (53 different outcomes) up to 180 days after each qHPV vaccine dose was observed. There was no association between exposure to qHPV vaccine and venous thromboembolism (RR 0.86; 95% CI 0.55–1.36). Regarding neurological events, the relative risk for five occurrences were not significantly increased, and there were inverse associations with epilepsy (RR 0.66; 95% CI 0.54–0.80) and paralysis (RR 0.56; 95% CI 0.35–0.90). However, with respect to autoimmune events exposure to qHPV vaccine was significantly associated with Behcet's syndrome (RR 3.37; 95% CI 1.05–10.80), Raynaud's disease (RR 1.67; 95 CI 1.14–2.44), and type 1 diabetes (RR 1.29; 95% CI 1.03–1.62). These findings corroborate those from a cohort study of 189,629 women in two managed care organizations in California, who found no safety signal when investigating the risk of 16 autoimmune events.15 That study did find an inverse association between exposure to qHPV vaccine and type 1 diabetes (RR 0.57; 95% CI 0.47–0.73), lending further support to the conclusion that the initial signal for type 1 diabetes that was seen in our study might be a false positive.36
Nevertheless, our results and the latter findings need to be confirmed in studies with longer follow-up time, validation of results, and data on time of onset of disease. Further monitoring of HPV vaccine safety is warranted in other populations where use and coverage have increased.
Despite these interesting data, there are several limitations to the evidence presented here. The heterogeneity of studies associated with methodological diversity would indicate that the studies suffer from different degrees of bias that prevented the conducting of a meta-analysis. Concerning AE, more studies evaluating both vaccines are still necessary.
Nonetheless, safety is not a major barrier to vaccination. The cost of vaccines is still the main limiting factor for their use in developing countries.
Conflicts of interestThe authors declare no conflicts of interest.