Dolutegravir (DTG) is amongst the most prescribed antiretrovirals worldwide and is recommended as first line regimen in most HIV treatment guidelines. Its use, although infrequently, had been associated to an increased chance of neural tube defects (NTD) in Botswana, Africa. Herein we describe two cases of NTD in women who conceived while taking DTG as part of their antiretroviral treatment in the city of Porto Alegre, Brazil.
Dolutegravir (DTG), a second-generation integrase inhibitor, is amongst the most prescribed antiretrovirals worldwide. It is included in international guidelines as a preferred first-line drug for people living with HIV, including women of child-bearing potential.1–4 Nevertheless, its use had been associated, although infrequently, with an increased chance of neural tube defects (NTD) in women who conceived while taking this drug. In May 2018, an unplanned interim evaluation of an observational surveillance study of birth outcomes among pregnant women on antiretroviral therapy (ART) in Botswana have shown an increased frequency (0.94%) in NTD in infants born to women who became pregnant while on DTG based regimen.5 This data had been recently updated and infants who were exposed to the drug around the time of conception had still higher chance of NTD than other groups (other ART and HIV negative women), although the risk was much lower than initially reported.6
Herein we report two cases of NTD in women with unplanned pregnancies who conceived while taking DTG in the city of Porto Alegre, Brazil.
Case reportsCase 1A 32-year-old Caucasian woman, with HIV diagnosis since 2007, with three previous pregnancies (including one spontaneous abortion) looked for pre-natal care after finding out she was pregnant.
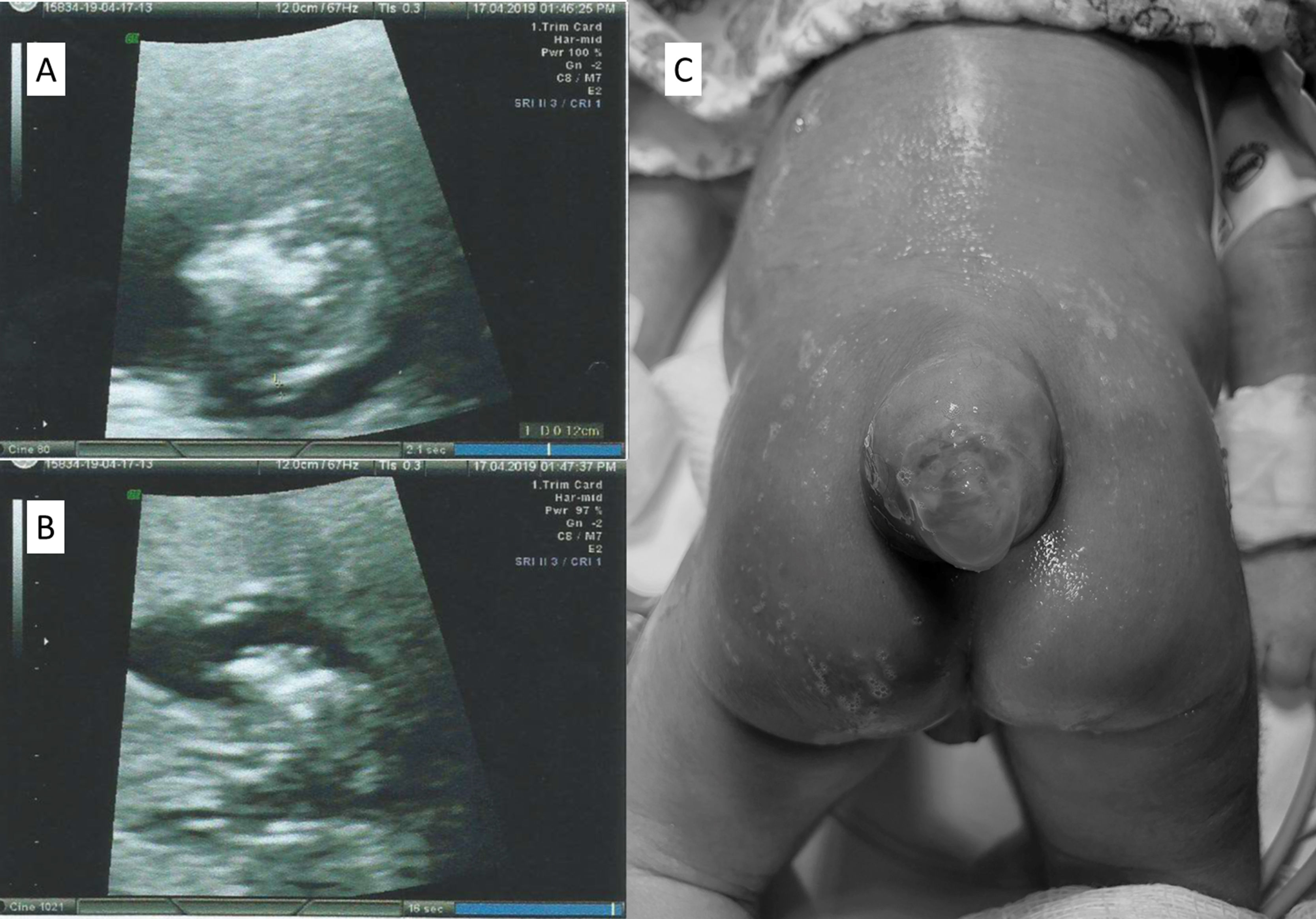
In December 2017 her ARV was switched for DTG, tenofovir (TDF), and lamivudine (3TC). Upon arriving at the pre-natal clinic, in February 2019, with an estimated seven weeks of pregnancy, DTG was switched for raltegravir (RAL). She had no history of folic acid supplementation at conception and it was introduced only after her initial appointment. HBV, HCV, syphilis, and toxoplasmosis tests yielded negative results. Lymphocyte T-CD4 count was 838 cells/mm3, and her HIV viral load (VL) was not detected. She never took other medicines besides ART. There was no history of diabetes mellitus, and her BMI was 28.3 kg/m2. She denied consumption of alcohol, tabaco or recreational drugs use. No family history of birth defect was referred. A first trimester morphologic ultrasound detected a 13-week pregnancy single-live fetus with absence of skull vault, compatible with anencephaly (Fig. 1). The family was counselled and offered pregnancy cessation, to which they agreed. She was referred to the hospital to perform an elective abortion. After the procedure anatomical pathology confirmed anencephaly in a male fetus, without other abnormalities. After pregnancy termination, and according to the patient request, contraceptive implant was inserted subcutaneously.
Case 2A 24-year-old African descendant woman with a recent HIV diagnosis, started DTG, TDF, and 3TC in May 2017. She looked for pre-natal care in January 2019, soon after her pregnancy disclosure, with a gestational age compatible to 14 weeks. At that time, DTG was switched for RAL. There was no use of folic acid at conception. HBV, HCV, syphilis, and toxoplasmosis tests yielded negative results. Lymphocyte T-CD4 count was 814 cells/mm3, and HIV VL was undetectable. There was no diabetes mellitus, and the BMI was 29.0 kg/m2. There was no history of anti-epileptic (including sodium valproate) or other medication besides ARVs; no alcohol, tabaco or recreational drugs use. No family history of birth defect. Membrane rupture occurred at 35 weeks of pregnancy and the patient was submitted to cesarean section as her VL at that time was unknown. Intravenous zidovudine was administered prior to the cesarean. A live male newborn was delivered weighting 1760 g with an apgar score of 8 and 9 at 1 and 5 min, respectively. The presence of spina bifida with membrane sac rupture was diagnosed (Fig. 1). The newborn received prophylactic oral zidovudine and nevirapine and was submitted to surgery for spina bifida 24 h after birth. The surgery undergone without complications and the baby remained at the hospital for one month. Cranial ultrasonography performed seven days after surgery demonstrated both lateral ventricles increased, probably related to Chiari malformation, type 2. Infant VL remained undetectable in the follow-up period, therefore with no vertical transmission of HIV infection. Neurological exam after one year did not reveal any abnormalities.
DiscussionTo our knowledge these are the first cases outside Tsepamo study of NTDs in women who became pregnant while on DTG. NTDs are among the most common birth defects worldwide with a prevalence ranging from 0.5 to more than 10 per 1000 pregnancies. This wide range likely reflects the different contributions from risk factors known to be associated to NTD such as nutritional status, prevalence of maternal factors, folic acid supplementation and/or fortification, presence of environmental toxicants, and genetic predisposition among ethnic groups.7 While maternal obesity and diabetes mellitus are recognized factors for NTD, drugs such as valproic acid and other chemotherapeutic agents have also been described.8
Deficient folic acid at conception until neural tube closure (generally at six weeks) is thought to be the core of NTD.9 This fact is the basic rationale for folic acid fortification and/or supplementation worldwide for women who intend to get pregnant. Some experimental “in vitro” and in animal studies, have found that exceptionally very high concentrations of DTG could impair the transplacental transport of folic acid.10 Nevertheless, these concentrations are highly improbable to be reached in humans. The risk of NTDs decreases after early pregnancy, though it is not clear exactly when this period of increased risk ends. Neural tube closes approximately four weeks post-conception, or approximately six weeks after the last menstrual period in women with regular menses. Therefore, the risk for a drug to cause NTDs is over by approximately six weeks gestational age. On the other hand, it is known that some human genetic polymorphisms might, on rare occasions, interfere with embryo folic acid offer, contributing as well to the possibility of NTD.7,8 All variables together could further increase, albeit still extremely low, the chance of NTD.
An update of Tsepamo study, comprising almost 200,000 pregnancies has been recently presented.6 Prevalence of NTDs in the comparator exposure group of non-DTG ART at conception was 0.11% (95% CI 0.07–0.17). The prevalence for other exposure groups was 0.07% for EFV at conception; 0.04% for DTG started in pregnancy; and 0.07% in HIV negative women. Prevalence difference between DTG and non-DTG ARVs at conception was 0.09% (95% CI −0.03% to 0.30%). This has also decreased since the last report as has the difference across all other exposure groups. In this scenario, considering the socio-economical differences worldwide WHO reinforced that the clinical benefits of using DTG outweighs (in relation to HIV transmission and death) any potential risks.2
The Antiretroviral Pregnancy Registry (it is important to notice that 75% of the data in the registry was from North America, Europe, and Latin America, where most countries adopt folate fortification for food), has not observed an increased risk of NTD with DTG.11 Likewise, a retrospective study performed by the Brazilian Ministry of Health in women with periconception ARV drug exposure showed no NTDs among 384 pregnancies in which infants were exposed to DTG.12
It is important to note that Brazil's mandatory folic acid fortification (FAF) policy has been implemented since 2004 but the dosage of 0.15 mg/100 g is lower than that used in other Latin American countries (0.22 mg/100 g). Brazil has the lower estimated consumption of wheat -flour-bread and, the reduction of NTDs, especially Spina Bifida, between 1982 and 2007, was lower in Brazil compared to other South American countries (Chile, Argentina and Uruguay).13
In an ideal clinical setting, clinicians should discuss future reproductive plans and timing as well as the risks and benefits of conceiving on specific ARV medications and use of appropriate contraceptive options to prevent unintended pregnancy. DTG might be prescribed to women of childbearing potential and not using or accessing consistent effective contraception if they are informed of the small but potential increased risk of NTD (at conception until the end of first trimester). When this risk is not acceptable by the woman an alternative regimen should be offered.
In summary, we reported two cases of NTD occurred in unplanned pregnancies from women on DTG as part of the antiretroviral regimen. Although still to be proven, the risk seems to be extremely low. Folic acid is known to prevent NTDs in the general population and women living with HIV who are pregnant or wishing to conceive should take folic acid daily supplement.
Conflicts of interestThe authors declare no conflicts of interest.
FundingThis work did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
We thank Dr. Rafael Silva Paglioli and Dr. Marcelo Castellano de Almeida for gently providing the imagens presented at this manuscript.