Diabetes mellitus (DM) has important implications for tuberculosis (TB), as it increases the risk for disease activation and is associated with unfavorable TB treatment outcomes. This study analyzed the association between TB and DM (TBDM) in Brazil from 2007 to 2014. This was a retrospective cohort study carried out in 709,429 new cases of TB reported to the national disease notification system of the Brazilian Ministry of Health. Sociodemographic and clinical data, test results, and treatment outcomes were analyzed. TBDM was found in 6.0% of TB cases, mostly in men aged 18-59 years. The lethality rate was 5.1% higher in all age groups with diabetes, except in those older than 60 years of age. The frequency of multi-drug-resistant tuberculosis (MDR-TB) in patients with DM was higher in those without DM, with a 1.6- to 3.8-fold increase in the odds of MDR-TB. The elderly showed an increase in the prevalence of TBDM from 14.3% to 18.2%. Women were more likely to have DM, and elderly women had 41.0% greater chance of having DM. Relapse was significant among patients younger than 17 years of age. TBDM was high in Brazil, affected all age groups, and was associated with unfavorable TB treatment outcomes. We emphasize the need for strategies for the clinical management of diabetic tuberculosis patients in Brazil aiming at minimizing relapses, deaths, and MDR-TB.
Diabetes mellitus (DM) has important implications for tuberculosis (TB), as it increases the risk for disease activation and results in unfavorable TB treatment outcomes.1,2 Both type 1 and type 2 DM are impact severity, clinical presentation, morbidity, mortality,3 and treatment failure of TB.1 For Cameron and Wherrett (2015),4 type 1 DM is a common chronic disorder in childhood or adolescence.3,5 However, with the increasing rates of childhood obesity and genetic forms of DM in children, type 2 DM has also increased in adolescents, young people, and adults.3 In addition, type 2 DM is seen as a progressive and severe condition in adolescents, who respond poorly to treatment, present with microvascular and macrovascular complications,6,7 and psychological and physical damage caused by obesity,5 causing additional costs to health systems.8
Moreover, patients with type 2 DM are likely to remain sputum-positive after two months of anti-TB treatment,9 have increased bilateral lung involvement, increased lymph nodes, and a high risk for serious adverse events.10 Additionally, the lack of glycemic control leads to severe and advanced forms of TB, with pulmonary cavitation, positive sputum, and slow smear and culture conversion during treatment.3,4,11 In these cases, greater drug resistance is evidenced (mono-resistance, poly-resistance, and multidrug resistance),10,12 in addition to unfavorable treatment outcome and frequent relapse.2 Although men and women are at risk for developing TBDM, men9 are more likely to develop TBDM. Some associated risk factors include failure in glycemic control,13 being older than 40,14 TB contacts,13 smoking,12 sedentary lifestyle,15 and high body weight.14,16
A systematic review showed a global prevalence of DM among patients with TB of 4.1%; in South America it ranged from 6.1% in Brazil to 14.0% in Guyana.15 Regarding age range, southern Nigeria showed a TBDM prevalence of 2.2% in individuals younger than 25 years of age, 16.9% in those between 56 and 65 years, and 11.5% in patients aged 65 and above.14 In conclusion, studies indicate that patients with DM have a two to three times greater risk of having TB than those without DM,17 and that type 2 DM accounts for more than 90.0% of this association.8
In view of the evidence, it was important to know the clinical-epidemiological situation of reported cases of TBDM by age group in Brazil. It was anticipated that the knowledge about TBDM would stimulate collaboration between TB and DM programs, and also allow the increase of preventive treatment in children with DM infected with TB bacilli. Another objective of disseminating the findings of this study was to promote adequate clinical management of TBDM cases, which is fundamental for eradicating TB in Brazil.
MethodThis was a retrospective cohort study with 709,429 new cases of TB reported in Brazil between January 2007 and December 2014. All cases were notified to the national disease notification system (Sistema de Informação de Agravos de Notificação – SINAN-TB) of the Brazilian Ministry of Health. The option to use the information from 2007 is justified by greater completeness of the variable “Associated Diseases” in the TB notification form. All the TB cases were included in the study, regardless of age, skin color/race, schooling, sputum smear and culture results, HIV results, clinical form, and treatment outcome.
“TB case” was classified according to the recommendations of the National Program for TB Control of the Brazilian Ministry of Health, and “DM case” according to the WHO guidelines.18,19 For TB diagnosis, Ziehl-Neelsen staining and culture in Lowenstein-Jensen solid medium were used.18 All participants received anti-TB treatment with rifampicin, pyrazinamide, and isoniazid for two months – in 2009 ethambutol was added to this regimen. Rifampicin-isoniazid was maintained in both regimens for the last 4 months.18
Statistical analyses were performed using Pearson's chi-squared test (χ2) to evaluate the frequency of the considered variables in different samples of patients. Due to the linear behavior of the annual proportions by age group, we used the linear regression model to evaluate any significant variation in the second year of analysis. Logistic regression analysis was used to estimate the independent association among TBDM and epidemiological and clinical variables. In all analyses, a significance level of 0.05 and a 95% confidence interval (95% CI) were considered. STATA software was used for statistical analyses. The research was approved by the Ethics Committee of the FEPESC/SES-DF (Document 0019/2011).
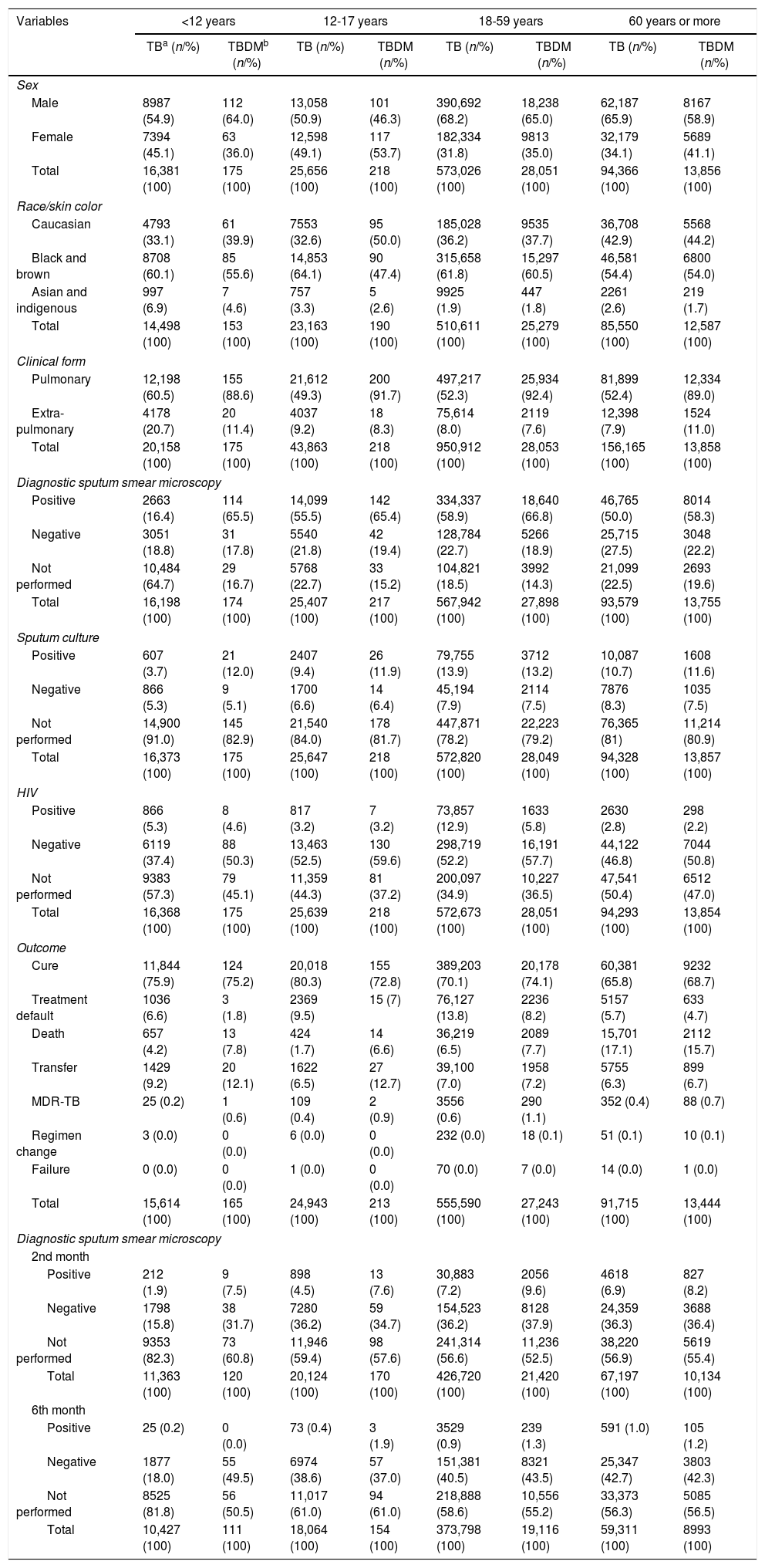
ResultsDM was diagnosed in 6.0% of TB cases reported in Brazil between 2007 and 2014, varying from 0.4% in those younger than 12 years of age, 0.5% in 12-17 years, 66.3% in 18-59 years, and 32.8% in 60 years or older. The general characteristics of the 709,429 TB cases and 42,306 TBDM are shown in Table 1. The mean age for TBDM was 40 years – interquartile range (IQR)=51-27) – and the majority were men (66.9%). According to age groups, TBDM was more frequent among the elderly (14.3-18.2%). There was an increasing tendency of TBDM in women in the last of the study period. Adolescent women (12-17 years of age) with TBDM outnumbered adolescent men. Blacks and brown-skinned were more associated with TBDM (mean of 58.3%), except among adolescents (Table 1). People with less than nine years of schooling were more affected by TBDM (83.8%).
Characteristics of patients with tuberculosis by age group and diagnosis of diabetes, Brazil, 2007-2014.
| Variables | <12 years | 12-17 years | 18-59 years | 60 years or more | ||||
|---|---|---|---|---|---|---|---|---|
| TBa (n/%) | TBDMb (n/%) | TB (n/%) | TBDM (n/%) | TB (n/%) | TBDM (n/%) | TB (n/%) | TBDM (n/%) | |
| Sex | ||||||||
| Male | 8987 (54.9) | 112 (64.0) | 13,058 (50.9) | 101 (46.3) | 390,692 (68.2) | 18,238 (65.0) | 62,187 (65.9) | 8167 (58.9) |
| Female | 7394 (45.1) | 63 (36.0) | 12,598 (49.1) | 117 (53.7) | 182,334 (31.8) | 9813 (35.0) | 32,179 (34.1) | 5689 (41.1) |
| Total | 16,381 (100) | 175 (100) | 25,656 (100) | 218 (100) | 573,026 (100) | 28,051 (100) | 94,366 (100) | 13,856 (100) |
| Race/skin color | ||||||||
| Caucasian | 4793 (33.1) | 61 (39.9) | 7553 (32.6) | 95 (50.0) | 185,028 (36.2) | 9535 (37.7) | 36,708 (42.9) | 5568 (44.2) |
| Black and brown | 8708 (60.1) | 85 (55.6) | 14,853 (64.1) | 90 (47.4) | 315,658 (61.8) | 15,297 (60.5) | 46,581 (54.4) | 6800 (54.0) |
| Asian and indigenous | 997 (6.9) | 7 (4.6) | 757 (3.3) | 5 (2.6) | 9925 (1.9) | 447 (1.8) | 2261 (2.6) | 219 (1.7) |
| Total | 14,498 (100) | 153 (100) | 23,163 (100) | 190 (100) | 510,611 (100) | 25,279 (100) | 85,550 (100) | 12,587 (100) |
| Clinical form | ||||||||
| Pulmonary | 12,198 (60.5) | 155 (88.6) | 21,612 (49.3) | 200 (91.7) | 497,217 (52.3) | 25,934 (92.4) | 81,899 (52.4) | 12,334 (89.0) |
| Extra-pulmonary | 4178 (20.7) | 20 (11.4) | 4037 (9.2) | 18 (8.3) | 75,614 (8.0) | 2119 (7.6) | 12,398 (7.9) | 1524 (11.0) |
| Total | 20,158 (100) | 175 (100) | 43,863 (100) | 218 (100) | 950,912 (100) | 28,053 (100) | 156,165 (100) | 13,858 (100) |
| Diagnostic sputum smear microscopy | ||||||||
| Positive | 2663 (16.4) | 114 (65.5) | 14,099 (55.5) | 142 (65.4) | 334,337 (58.9) | 18,640 (66.8) | 46,765 (50.0) | 8014 (58.3) |
| Negative | 3051 (18.8) | 31 (17.8) | 5540 (21.8) | 42 (19.4) | 128,784 (22.7) | 5266 (18.9) | 25,715 (27.5) | 3048 (22.2) |
| Not performed | 10,484 (64.7) | 29 (16.7) | 5768 (22.7) | 33 (15.2) | 104,821 (18.5) | 3992 (14.3) | 21,099 (22.5) | 2693 (19.6) |
| Total | 16,198 (100) | 174 (100) | 25,407 (100) | 217 (100) | 567,942 (100) | 27,898 (100) | 93,579 (100) | 13,755 (100) |
| Sputum culture | ||||||||
| Positive | 607 (3.7) | 21 (12.0) | 2407 (9.4) | 26 (11.9) | 79,755 (13.9) | 3712 (13.2) | 10,087 (10.7) | 1608 (11.6) |
| Negative | 866 (5.3) | 9 (5.1) | 1700 (6.6) | 14 (6.4) | 45,194 (7.9) | 2114 (7.5) | 7876 (8.3) | 1035 (7.5) |
| Not performed | 14,900 (91.0) | 145 (82.9) | 21,540 (84.0) | 178 (81.7) | 447,871 (78.2) | 22,223 (79.2) | 76,365 (81) | 11,214 (80.9) |
| Total | 16,373 (100) | 175 (100) | 25,647 (100) | 218 (100) | 572,820 (100) | 28,049 (100) | 94,328 (100) | 13,857 (100) |
| HIV | ||||||||
| Positive | 866 (5.3) | 8 (4.6) | 817 (3.2) | 7 (3.2) | 73,857 (12.9) | 1633 (5.8) | 2630 (2.8) | 298 (2.2) |
| Negative | 6119 (37.4) | 88 (50.3) | 13,463 (52.5) | 130 (59.6) | 298,719 (52.2) | 16,191 (57.7) | 44,122 (46.8) | 7044 (50.8) |
| Not performed | 9383 (57.3) | 79 (45.1) | 11,359 (44.3) | 81 (37.2) | 200,097 (34.9) | 10,227 (36.5) | 47,541 (50.4) | 6512 (47.0) |
| Total | 16,368 (100) | 175 (100) | 25,639 (100) | 218 (100) | 572,673 (100) | 28,051 (100) | 94,293 (100) | 13,854 (100) |
| Outcome | ||||||||
| Cure | 11,844 (75.9) | 124 (75.2) | 20,018 (80.3) | 155 (72.8) | 389,203 (70.1) | 20,178 (74.1) | 60,381 (65.8) | 9232 (68.7) |
| Treatment default | 1036 (6.6) | 3 (1.8) | 2369 (9.5) | 15 (7) | 76,127 (13.8) | 2236 (8.2) | 5157 (5.7) | 633 (4.7) |
| Death | 657 (4.2) | 13 (7.8) | 424 (1.7) | 14 (6.6) | 36,219 (6.5) | 2089 (7.7) | 15,701 (17.1) | 2112 (15.7) |
| Transfer | 1429 (9.2) | 20 (12.1) | 1622 (6.5) | 27 (12.7) | 39,100 (7.0) | 1958 (7.2) | 5755 (6.3) | 899 (6.7) |
| MDR-TB | 25 (0.2) | 1 (0.6) | 109 (0.4) | 2 (0.9) | 3556 (0.6) | 290 (1.1) | 352 (0.4) | 88 (0.7) |
| Regimen change | 3 (0.0) | 0 (0.0) | 6 (0.0) | 0 (0.0) | 232 (0.0) | 18 (0.1) | 51 (0.1) | 10 (0.1) |
| Failure | 0 (0.0) | 0 (0.0) | 1 (0.0) | 0 (0.0) | 70 (0.0) | 7 (0.0) | 14 (0.0) | 1 (0.0) |
| Total | 15,614 (100) | 165 (100) | 24,943 (100) | 213 (100) | 555,590 (100) | 27,243 (100) | 91,715 (100) | 13,444 (100) |
| Diagnostic sputum smear microscopy | ||||||||
| 2nd month | ||||||||
| Positive | 212 (1.9) | 9 (7.5) | 898 (4.5) | 13 (7.6) | 30,883 (7.2) | 2056 (9.6) | 4618 (6.9) | 827 (8.2) |
| Negative | 1798 (15.8) | 38 (31.7) | 7280 (36.2) | 59 (34.7) | 154,523 (36.2) | 8128 (37.9) | 24,359 (36.3) | 3688 (36.4) |
| Not performed | 9353 (82.3) | 73 (60.8) | 11,946 (59.4) | 98 (57.6) | 241,314 (56.6) | 11,236 (52.5) | 38,220 (56.9) | 5619 (55.4) |
| Total | 11,363 (100) | 120 (100) | 20,124 (100) | 170 (100) | 426,720 (100) | 21,420 (100) | 67,197 (100) | 10,134 (100) |
| 6th month | ||||||||
| Positive | 25 (0.2) | 0 (0.0) | 73 (0.4) | 3 (1.9) | 3529 (0.9) | 239 (1.3) | 591 (1.0) | 105 (1.2) |
| Negative | 1877 (18.0) | 55 (49.5) | 6974 (38.6) | 57 (37.0) | 151,381 (40.5) | 8321 (43.5) | 25,347 (42.7) | 3803 (42.3) |
| Not performed | 8525 (81.8) | 56 (50.5) | 11,017 (61.0) | 94 (61.0) | 218,888 (58.6) | 10,556 (55.2) | 33,373 (56.3) | 5085 (56.5) |
| Total | 10,427 (100) | 111 (100) | 18,064 (100) | 154 (100) | 373,798 (100) | 19,116 (100) | 59,311 (100) | 8993 (100) |
Source: National disease notification system (Sistema de Informação de Agravos de Notificação – SINAN). Chi-square test, p<0.001.
The pulmonary form of TB prevailed (89.0%). Relapse rate was 6.5%. In all age groups, 64.0% were sputum smear positive, and 8.2% and 1.6% of cases remained positive in the 2nd and 4th month of treatment, respectively. By the 6th month of treatment, on average, 1% of TBDM patients had positive smears, compared to 0% in those younger than 12 years of age (p<0.001). Moreover, 12.7% were sputum culture positive, compared to 13.2% in the age group 18-59 years. All comparisons were statistically significant (p<0.001) (Table 1).
The mean TB cure rate was 72.7%. The proportion of HIV positive was generally lower in those with DM than without DM; in contrast, death rate was higher in TBDM of all age groups, except in those aged 60 years or older (Table 1). MDR-TB was 0.8% in TBDM and was higher in all age groups compared to those without DM.
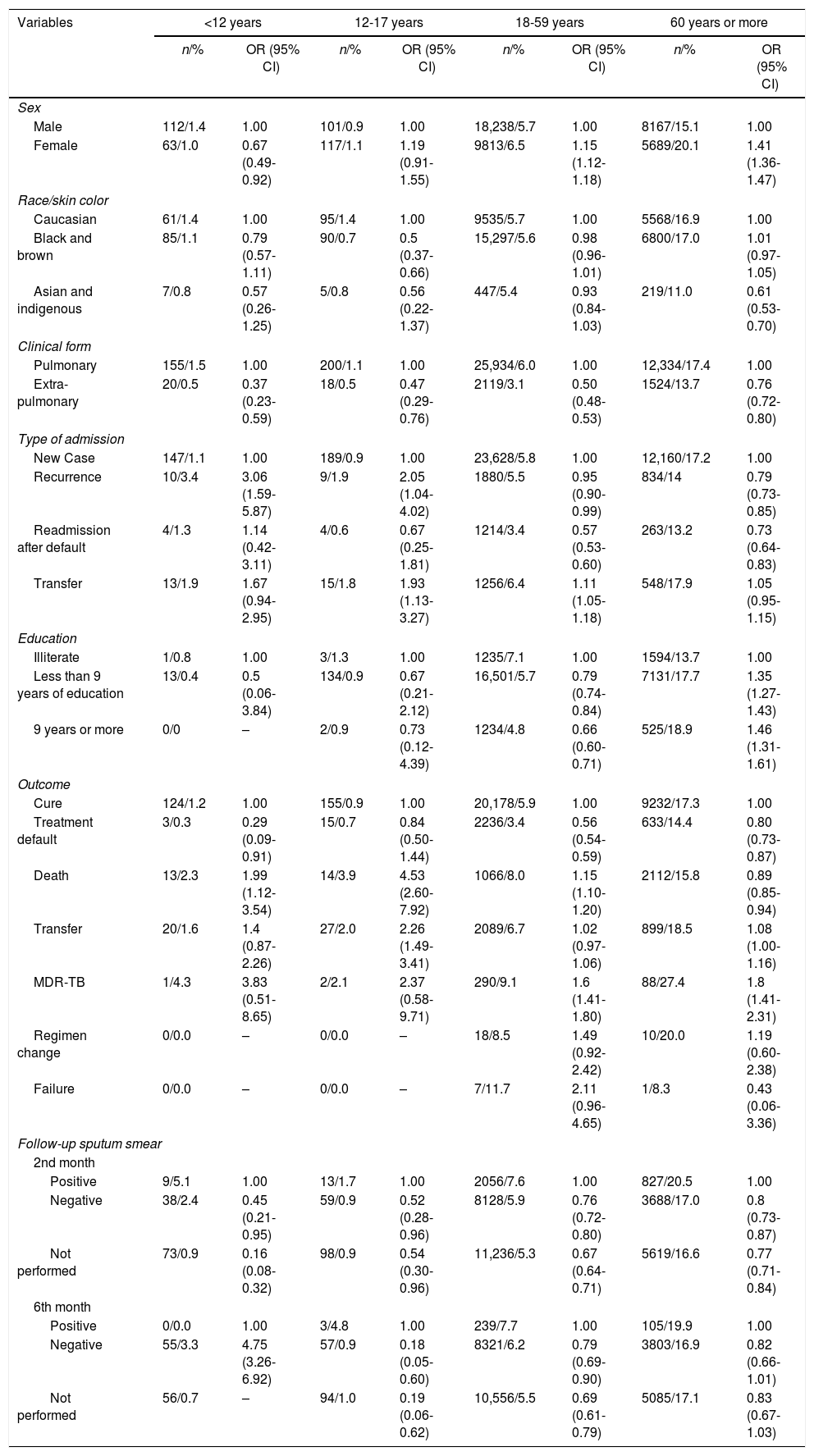
Women were more likely to be affected by DM than men, except in those aged less than 12 years of age, suggesting that older women were more likely to have DM. Among the elderly, the chance of TBDM occurrence increased by 41.0% in women. Black and brown adolescents showed a 50.0% reduced chance of TBDM when compared to whites. The chance of TBDM increased with each year of education. In the age group 60 years or more, smear microscopy during follow-up in the 2nd month was shown to be less likely to be negative (92%), and in the 6th month, the odds of being positive were 82% and 21% in adolescents and adults, respectively. TBDM relapse in those aged less than 12 years was three times greater when compared to a new case, and in adolescents, the chance increased more than twofold. In people aged 18 years or over, relapse was a protective factor (Table 2).
Proportion of the association between TBDM and associated risk factors, Brazil, 2007-2014.
| Variables | <12 years | 12-17 years | 18-59 years | 60 years or more | ||||
|---|---|---|---|---|---|---|---|---|
| n/% | OR (95% CI) | n/% | OR (95% CI) | n/% | OR (95% CI) | n/% | OR (95% CI) | |
| Sex | ||||||||
| Male | 112/1.4 | 1.00 | 101/0.9 | 1.00 | 18,238/5.7 | 1.00 | 8167/15.1 | 1.00 |
| Female | 63/1.0 | 0.67 (0.49-0.92) | 117/1.1 | 1.19 (0.91-1.55) | 9813/6.5 | 1.15 (1.12-1.18) | 5689/20.1 | 1.41 (1.36-1.47) |
| Race/skin color | ||||||||
| Caucasian | 61/1.4 | 1.00 | 95/1.4 | 1.00 | 9535/5.7 | 1.00 | 5568/16.9 | 1.00 |
| Black and brown | 85/1.1 | 0.79 (0.57-1.11) | 90/0.7 | 0.5 (0.37-0.66) | 15,297/5.6 | 0.98 (0.96-1.01) | 6800/17.0 | 1.01 (0.97-1.05) |
| Asian and indigenous | 7/0.8 | 0.57 (0.26-1.25) | 5/0.8 | 0.56 (0.22-1.37) | 447/5.4 | 0.93 (0.84-1.03) | 219/11.0 | 0.61 (0.53-0.70) |
| Clinical form | ||||||||
| Pulmonary | 155/1.5 | 1.00 | 200/1.1 | 1.00 | 25,934/6.0 | 1.00 | 12,334/17.4 | 1.00 |
| Extra-pulmonary | 20/0.5 | 0.37 (0.23-0.59) | 18/0.5 | 0.47 (0.29-0.76) | 2119/3.1 | 0.50 (0.48-0.53) | 1524/13.7 | 0.76 (0.72-0.80) |
| Type of admission | ||||||||
| New Case | 147/1.1 | 1.00 | 189/0.9 | 1.00 | 23,628/5.8 | 1.00 | 12,160/17.2 | 1.00 |
| Recurrence | 10/3.4 | 3.06 (1.59-5.87) | 9/1.9 | 2.05 (1.04-4.02) | 1880/5.5 | 0.95 (0.90-0.99) | 834/14 | 0.79 (0.73-0.85) |
| Readmission after default | 4/1.3 | 1.14 (0.42-3.11) | 4/0.6 | 0.67 (0.25-1.81) | 1214/3.4 | 0.57 (0.53-0.60) | 263/13.2 | 0.73 (0.64-0.83) |
| Transfer | 13/1.9 | 1.67 (0.94-2.95) | 15/1.8 | 1.93 (1.13-3.27) | 1256/6.4 | 1.11 (1.05-1.18) | 548/17.9 | 1.05 (0.95-1.15) |
| Education | ||||||||
| Illiterate | 1/0.8 | 1.00 | 3/1.3 | 1.00 | 1235/7.1 | 1.00 | 1594/13.7 | 1.00 |
| Less than 9 years of education | 13/0.4 | 0.5 (0.06-3.84) | 134/0.9 | 0.67 (0.21-2.12) | 16,501/5.7 | 0.79 (0.74-0.84) | 7131/17.7 | 1.35 (1.27-1.43) |
| 9 years or more | 0/0 | – | 2/0.9 | 0.73 (0.12-4.39) | 1234/4.8 | 0.66 (0.60-0.71) | 525/18.9 | 1.46 (1.31-1.61) |
| Outcome | ||||||||
| Cure | 124/1.2 | 1.00 | 155/0.9 | 1.00 | 20,178/5.9 | 1.00 | 9232/17.3 | 1.00 |
| Treatment default | 3/0.3 | 0.29 (0.09-0.91) | 15/0.7 | 0.84 (0.50-1.44) | 2236/3.4 | 0.56 (0.54-0.59) | 633/14.4 | 0.80 (0.73-0.87) |
| Death | 13/2.3 | 1.99 (1.12-3.54) | 14/3.9 | 4.53 (2.60-7.92) | 1066/8.0 | 1.15 (1.10-1.20) | 2112/15.8 | 0.89 (0.85-0.94) |
| Transfer | 20/1.6 | 1.4 (0.87-2.26) | 27/2.0 | 2.26 (1.49-3.41) | 2089/6.7 | 1.02 (0.97-1.06) | 899/18.5 | 1.08 (1.00-1.16) |
| MDR-TB | 1/4.3 | 3.83 (0.51-8.65) | 2/2.1 | 2.37 (0.58-9.71) | 290/9.1 | 1.6 (1.41-1.80) | 88/27.4 | 1.8 (1.41-2.31) |
| Regimen change | 0/0.0 | – | 0/0.0 | – | 18/8.5 | 1.49 (0.92-2.42) | 10/20.0 | 1.19 (0.60-2.38) |
| Failure | 0/0.0 | – | 0/0.0 | – | 7/11.7 | 2.11 (0.96-4.65) | 1/8.3 | 0.43 (0.06-3.36) |
| Follow-up sputum smear | ||||||||
| 2nd month | ||||||||
| Positive | 9/5.1 | 1.00 | 13/1.7 | 1.00 | 2056/7.6 | 1.00 | 827/20.5 | 1.00 |
| Negative | 38/2.4 | 0.45 (0.21-0.95) | 59/0.9 | 0.52 (0.28-0.96) | 8128/5.9 | 0.76 (0.72-0.80) | 3688/17.0 | 0.8 (0.73-0.87) |
| Not performed | 73/0.9 | 0.16 (0.08-0.32) | 98/0.9 | 0.54 (0.30-0.96) | 11,236/5.3 | 0.67 (0.64-0.71) | 5619/16.6 | 0.77 (0.71-0.84) |
| 6th month | ||||||||
| Positive | 0/0.0 | 1.00 | 3/4.8 | 1.00 | 239/7.7 | 1.00 | 105/19.9 | 1.00 |
| Negative | 55/3.3 | 4.75 (3.26-6.92) | 57/0.9 | 0.18 (0.05-0.60) | 8321/6.2 | 0.79 (0.69-0.90) | 3803/16.9 | 0.82 (0.66-1.01) |
| Not performed | 56/0.7 | – | 94/1.0 | 0.19 (0.06-0.62) | 10,556/5.5 | 0.69 (0.61-0.79) | 5085/17.1 | 0.83 (0.67-1.03) |
Source: National disease notification system (Sistema de Informação de Agravos de Notificação – SINAN). Note: *logistic regression model.
TBDM increased the chance of the outcome “death” by two times when compared to cure in the age group <12 years, 4.5 times higher among adolescents. MDR-TB was relevant with an increase of 1.6- to 3.8-fold in all age groups (Table 2).
DiscussionThe proportion of DM cases reported among TB patients in this study was 6.0%, close to data from Nigeria (5.7%)14,20 and India (8.0%),21 which denotes the magnitude of this association in Brazil and the similarity of our results with the world literature, varying between 2.4% and 8.3%.22 It should be emphasized that our study analyzed only cases notified to the Ministry of Health. Therefore, there is a possibility of DM underreporting or reporting delay in Brazil, partly due to lack of established norms for identifying suspected DM, as there is no dialog between TB and DM programs. Thus, there no strategies in place for identifying DM among TB patients and vice versa, neither development of clinical and therapeutic management protocols TB cases with DM. A systematic review indicates that DM increases the risk of developing TB by about three times, varying from 0.99 to 7.83 in individual studies.23 A possible explanation for such variability may result from different retrospective analyses with less strict diagnostic criteria for DM, host genetic diversity, and influence of environmental factors on the immune response against TB,23 in addition to underreporting of the two associated morbidities. The increased risk of TB associated with DM generates an impact on public health due to increasing number of patients with DM in high burden TB24 areas with subsequent reactivation of latent cases.18
In the present study provides evidence of increased TBDM in children and adolescents, and particularly among adolescent women (12-17 years of age), who outnumbered men in the same age group. The literature indicates that adolescents are at risk of developing TBDM13 due to three factors: increased incidence of type 1 DM in childhood,3 recognition of increased type 2 DM and genetic forms of DM, and those aged between 10 and 24 years represent a quarter of the world population.4 Therefore, in order to face the new challenges posed by TBDM, behavioral and attitudinal changes of adolescents, health professionals, and society as whole are warranted. Yet, this is an opportune time for adolescents to gain confidence and ability to manage DM before adulthood.4,11 In addition, the fact that DM therapy requires lifestyle modification is another limiting factor for adolescents.4,6 Given the low adherence to treatment,3 adolescents with DM should use preventive strategies that help them overcome their psychosocial difficulties.5 Some of these strategies include controlling unhealthy diets and reducing sedentary lifestyle and obesity to cope with the double disease burden.18 The high prevalence of type 2 DM in early adolescence is relevant not only because it is a chronic clinical condition, but also due to its aggressive presentation in early adulthood.4,5,11
Considering sex, men and those aged 60 years or older were the most affected by TBDM. The latter showed a significant increase in TBDM in the study period from 14.3% to 18.2% (p<0.001). Reports from developed and developing countries show that men are at increased risk for developing TB,23,25 especially among the elderly.14,15,17 As the frequency of type 2 DM increases,11 men are more affected by the disease than women,17 as found in the present study. Moreover, the elderly present with greater frequency of positive smears and more clinical and treatment complications.26 In South Korea, a case-control study showed a higher frequency of men with cavitary disease and culture positivity in the 2nd month of therapy. In that study, DM was the only factor associated with unfavorable treatment outcomes.9 In Pakistan, TBDM affected more men in the age group 57 years,25 a result similar to the data obtained from Brazil. However, older people with less than nine years of schooling were more affected by TBDM.25 Differently, in Brazil, elderly with more than nine years of education showed a 1.46-fold greater risk of TBDM in comparison with illiterates. This discrepancy may be due to poor capacity of TB and DM programs to diagnosis DM among those with TB.
The present study showed that women were more likely to have TBDM than men, except those aged 12-17 years of age. In Mexico, a progressive sex-shift in prevalence from male to female was observed in patients diagnosed with pulmonary TBDM after 50 years of age; that is, there was a significant association between age and sex.27 However, women – despite the biological differences, lifestyle, obesity, and time spent caring for their children – usually seek more health care than men. Even so, it seems that at the time of TB diagnosis, history of DM is not inquired neither investigated lab tests, resulting in underreporting or late diagnosis of DM.
According to the present study, the risk of dying was almost five-fold higher in adolescents and more than two-fold in those aged less than 12 years. Studies have shown that children and adolescents with DM are at higher risk of death due to acute complications, including hypoglycemia, diabetic ketoacidosis, and high glucose intolerance, which were presented by almost twice as much by patients with TBDM.7 A systematic review by Baker et al. (2011)28 revealed that the risk of death from TB was 1.89 times higher in diabetics. Finally, particularly type 2 DM in adolescents appears to be more aggressive,11 causing microvascular and macrovascular complications and severe psychological and physical distress to affected people.8 The severity of the disease in this age group justifies the need for strict glycemic control due to the risk of severe hypoglycemia,11 and requires lifestyle interventions on young patients. We also observed that men with TBDM in most age groups had higher mortality than women. Although studies have shown deaths by TBDM in men and women at any age, the risk of dying is 3.6-24-fold greater in men.29 In Brazil, isolated DM revealed a sex-shift in predominance from female to male,30 and in case of TBDM, greater mortality rates than in the general population.1,2 Probably, the higher mortality rates are also explained by social and racial disparities.18 In contrast, a systematic review found no increased risk of death.23
TBDM in all age groups had a higher chance of developing MDR-TB. The main finding of the present study was the high failure rate in TB treatment predicted by failure of smear and culture conversion by the second month, which was also a risk factor for MDR-TB. Once again, we emphasize the importance of diagnosing or identifying DM early in patients with TB, as failure in glycemic control or clinical and therapeutic management during TB treatment may lead to drug resistance. A case-control study with hospitalized patients in Iran showed that 3.2% of TBDM had MDR-TB compared with no cases of MDR-TB in the control group and more TBDM cases had isolates that were resistant to at least one drug (12.9% vs. 10.9%).31 A study conducted in Tbilisi, Georgia, also highlighted the relationship between diabetes and MDR-TB.12 According to a meta-analysis, DM was also considered an independent risk factor for MDR-TB, especially primary MDR-TB.32 MDR-TB related death was high in Bangladesh.
Pulmonary TB was the most frequent form in this study, similar to the findings of Leung et al. (2017).1 Among cases of TBDM, we observed greater chance of recurrence in those aged less than 12 years and between 12 and 17 years (more than three times and more than two times greater, respectively); and the highest treatment failure rates in the age group 18-59 years (2.1 times greater). A study conducted by Beker et al. (2011)29 found a relapse rate of 3.9% and other study by Mi et al. (2013)23 showed a treatment failure rate of 10.3%. These results corroborate the findings of this study that points to high relapse and failure rates among TBDM patients in Brazil.
In TBDM cases, positive sputum smear was observed in almost 64.0% of the cases and prevailed in all age groups. Harries et al. (2013)18 showed that, among initially positive patients, 31.8% maintained positivity after the 2nd month of TB treatment regardless of the age group. In that study, smear at the 2nd, 4th, and 6th month of treatment remained more positive in TBDM than in those without DM, except in patients younger than 12 years, in line with the results of this series. In Riyadh, Saudi Arabia, during diagnosis, 65.2% of the TBDM group had positive sputum compared to 54.1% in the control group (p=0.008).33 It should be clarified that the justifications for late sputum conversion and treatment failure is based on interactions between oral hypoglycemic drugs and rifampicin, in addition to limited immune response due to DM.23 A study from South Korea reported delayed sputum conversion in TBDM patients,9 corroborating the findings in Brazil. People with TBDM and inadequate glycemic control tend to present with more advanced and severe clinical forms of TB, with cavitations and positive sputum smear results after the second month of treatment. Still, sputum smear conversion is slow, reducing the cure rate and increasing the chances of more prolonged treatment and recurrence in patients with pulmonary TB.2 In this series, on average, 12.7% of the participants had a positive sputum culture at diagnosis, the highest rate being observed in adolescents and in people aged 60 years or older. Leung et al. (2017)1 had reinforced that sputum culture remains positive after the second month of treatment and that the conversion time is longer among people with TBDM.12
Study limitationsRegarding study limitations, selection bias may have occurred, since the cases were classified according to the medical diagnosis performed at the patient's health care unit, that is, from routine data, secondary information, the SINAN database, and medical records. Although the data were reported to a specific TB section of SINAN (SINAN-TB), we believe there might have been underreporting of DM cases in Brazil.
ConclusionData regarding TBDM in this study are relevant. Although men account for a greater number of TBDM cases, adolescent women and older women showed a greater chance of having TBDM. We observed an increase in MDR-TB in all age groups among patients with TBDM. The evidence presented in our study points to the need to reduce the double burden of the disease in Brazil. Our suggestion is that this goal could be achieved through interventions in professional training for the clinical management of TBDM in health services.
Conflicts of interestThe authors declare no conflicts of interest.