Malnutrition is associated with an increased risk of complications in hospitalized patients, and parenteral nutrition (PN) is used when oral or enteral feeding is not possible. This study aimed at analyzing associations between PN characteristics and infectious complications in hospitalized patients.
Material and methodsThis was a retrospective cohort study conducted in a tertiarycare university hospital. Data from consecutive adult patients submitted to PN (January 2016 to December 2017; ICU and ward) were reviewed by means of an electronic database. Patient’s clinical characteristics, PN prescription and catheter insertion procedure data were extracted and analyzed. The main outcome was the development of central line–associated bloodstream infection (CLABSI). The secondary outcomes were other infectious complications and mortality, as well as factors associated with CLABSI.
ResultsWe analyzed 165 patients and 247 catheters used for parenteral nutrition infusion. The CLABSI rate was 6.47 per 1000 catheter-days. In the univariable analysis, CLABSI was associated with longer hospitalization time, longer PN time, longer catheter time, catheter insertion performed by a surgeon or a surgical resident, and procedures performed outside the ICU. In an extended time-dependent Cox regression, no variable was associated with a higher risk of CLABSI, and additional PN days did not increase the rate of CLABSI. The overall mortality rate was 24.8%. Only the patients’ comorbidity index was associated with death in the multivariable analysis.
DiscussionIn our study, patients who needed PN had an overall CLABSI rate of 6.47 per 1000 catheter-days. These outcomes were not associated with PN and catheter characteristics studied after adjustment for catheter time. The overall mortality rate was 24.8% and it was not associated with PN in multivariable analyses, only with Charlson comorbidity index.
Malnutrition is associated with an increased risk of complications, higher mortality rate, longer hospital stays, and higher hospitalization costs.1 Nutritional support is an alternative to overcome this problem, and it is indicated for patients unable to feed orally.2 There are two available options: enteral nutrition, usually chosen to preserve the patient’s gastrointestinal transit,3 and parenteral nutrition (PN), used when it is impossible to achieve partial or full enteral nutrition (EN) requirements. A pragmatic multicenter randomized clinical trial evaluated PN versus EN in ICU patients of developed countries and found no difference in both nutritional strategies in terms of number of treated infectious complications or 90-day mortality.4
The infection rate related to a central venous catheter (CVC) used for PN varies according to the definition used. This rate can reach up to 18 infectious events per 1000 catheter-days,5,6 a higher rate compared with central catheter infections in devices not used for PN (two infectious events per 1000 catheter-days in US intensive care units (ICUs) and 6.8 infectious events per 1000 catheter-days in developing countries' ICUs7). However, most central line infection data come from developed countries where resources differ (including the types of PN available and the device used for PN nutrition) from emerging countries.8 A multicentric Brazilian publication reported 10.22 bloodstream infections per 1000 catheters/day, and the risk factors for infection were multiple–lumen catheters, duration of catheterization and length of stay in the ICU, but PN was not evaluated as a variable in that study.9 Indeed, another study performed in the same country showed that PN was a risk factor for central venous catheter infection.10
Most studies on infection rates of PN refer to specific populations, such as critically ill, cancer or trauma patients.5 Few studies evaluated different diseases, non-critically-ill patients, catheter bundles and physicians experience to insert CVC, using a recently inserted versus an already used catheter for nutrition purposes. Studying this more heterogeneous cohort may infer more associations with the route of nutrition itself and not regarding specific groups. Furthermore, characteristics of the vascular access correlated with increased odds of infection in PN users are unknown, and the recommendations regarding the best vascular access to PN have low to very low quality of evidence.11 Therefore, the aim of the present study was to examine mediators of PN and central line–associated bloodstream infection (CLABSI) in a tertiary-care-level hospital. The secondary aim was to analyze the rate of other complications in patients submitted to PN.
Materials and methodsA retrospective cohort study was conducted in an 800-bed tertiary-care university hospital in the south of Brazil through review of electronic medical records of all adult inpatients submitted to PN in the period of January 2016 to December 2017. Patients who received PN for less than 72h were excluded from the analyses, as were those who received PN through a long-term catheter (LTC) due to their out-of-hospital use and possibility of lack of notification or even occurrence of an outcome in another institution. A peripherally inserted central catheter (PICC) were used in the hospital during the study only in experimental situations and they were not analyzed because of the possibility of bias due to differentiated care related to a new technology/device in the population. The study was approved by the local research ethics committee.
The assistant physician (based on local protocol and current guidelines) defined the choice for the total or supplementary PN.2,3 All of the prescribed solutions were two-in-one (2:1), combining glucose and amino acids, separately from intravenous lipid emulsion. The available solutions and the products used were Fresenius Kabi—Germany, Aminoven 10%, Lipovenos MCT 20%, and glucose 50%, with electrolytes, vitamin K, trace elements, and addition of multivitamins. Glycemic control during hospitalization was an attribution of the attending physician, as was the CVC installation, although both are standardized procedures. The local protocol about care with central lines includes qualified personnel and a bundle for best care of CVC.12 According to our hospital protocol, all physicians were encouraged to start enteral or oral diet and discontinue the PN solution as soon as possible and the device should be removed, since it would be no longer necessary.
Demographics characteristics, clinical data,13–15 and aspects of CVC insertion were reviewed. Daily records from the insertion of the first CVC used for PN until discharge or death were revised. Patients were classified according to the indication for PN: total PN, when there was contraindication or intolerance to any amount of enteral or oral diet or supplemental PN, when it was not possible to achieve the nutritional goal only with enteral or oral diet. For each patient, total hospitalization time, total PN time, total time with CVC in use, and time between the CVC insertion and start of PN were calculated.
The main outcome was development of central line–associated bloodstream infection (CLABSI), defined as CVC with clinical signs of infection and no other source of bacteremia, except the catheter up to 48h after the CVC’s withdrawal, plus 1) one positive blood culture for a known pathogen or 2) two positive blood cultures for skin pathogen.16
We also recorded as secondary outcomes other infections (pulmonary infection, abdominal infection, bacteremia not related to CVC, fungemia, urinary infection, operative wound infection based on clinical diagnosis), death, and hyperglycemia, as well as factors associated with CLABSI. Hyperglycemia was arbitrarily defined as at least four episodes of capillary glucose >200mg/dl during PN infusion; a need for a regular insulin prescription to achieve glycemic control; or a description of decompensated diabetes.
Sample size calculation, considering 18.3% cumulative incidence of CLABSI as found in a study performed in a similar population in the same hospital,10 was estimated in 231 catheters evaluation to identify factors associated with CVC infection, considering a power of 95% and a margin of error of 5%.
Statistic analyses were conducted as appropriated. Continuous variables were reported as mean and standard deviation, median and interquartile range, or number of patients and percentages. The differences between the groups were analyzed with Student’s t-test, Mann-Whitney U-test, or χ2, as appropriate. Generalized estimating equations were used for comparison in relation to CVC (more than one device per patient is possible). In multivariable analysis independent variables were included in the model according to their significance in the univariate analysis (p<0.05) or their biological importance. The results were expressed as hazard ratio (HR) with their respective 95% confidence intervals (CI). For analysis of CVC infection, Cox regression adjustments were performed for time-dependent covariables (CVC time in days) until the occurrence of the patient’s first event. The other catheters inserted after the occurrence of CLABSI were excluded from this analysis. The data were stored and analyzed in the statistical programs SPSS 22.0 (IBM SPSS Statistics for Windows, Armonk, NY) and R version 3.5.1 (Foundation for Statistical Computing, Vienna, Austria). In all analyses, a p-value <0.05 was considered as statistically significant. The study was conducted in accordance with local regulations and with the current guidelines for observational studies.17 All data were analyzed anonymously.
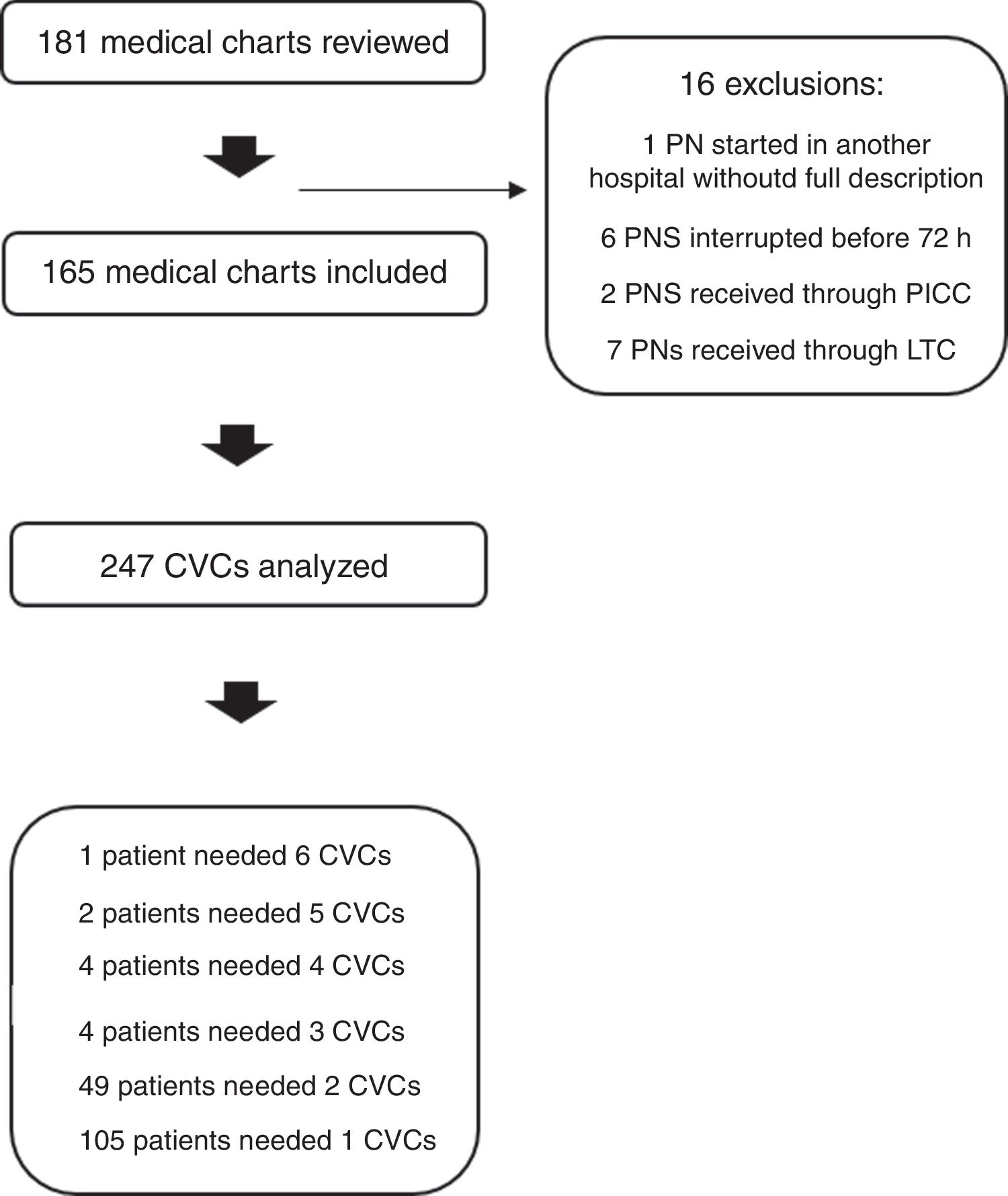
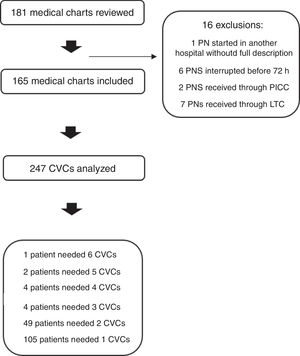
ResultsA total of 181 medical charts of patients who received PN between January 2016 and December 2017 (24 consecutive months) were reviewed. Sixteen patients were excluded leaving 165 patients and 247 CVCs (Fig. 1).
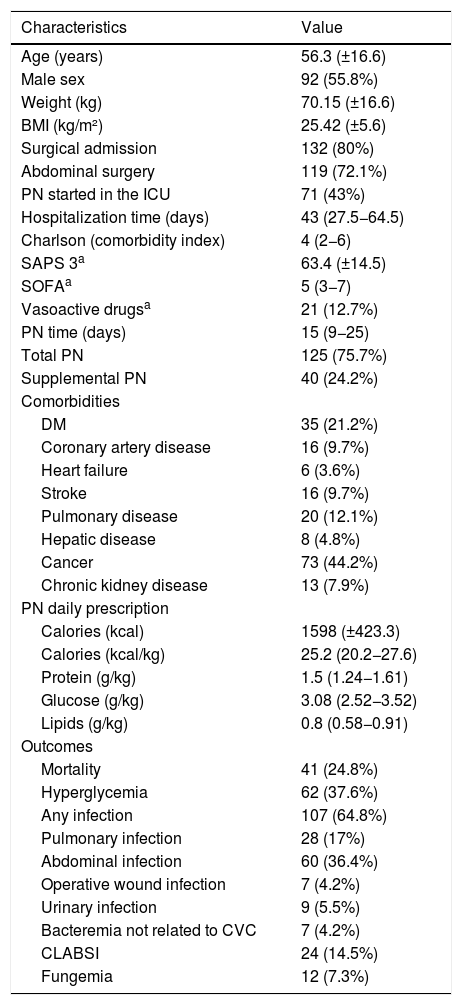
Table 1 summarizes the main characteristics of the included patients. Most patients were male, 56.3±16.6 years old, overweight, median Charlson index was 4, and the most frequent comorbidity was cancer. Mean nutritional prescription, caloric and proteic, was adequate. Overall mortality rate was 24.8%. The most prevalent outcome was any infectious complication during PN administration, mainly due to abdominal infection.
Characteristics of the included patients (n=165).
| Characteristics | Value |
|---|---|
| Age (years) | 56.3 (±16.6) |
| Male sex | 92 (55.8%) |
| Weight (kg) | 70.15 (±16.6) |
| BMI (kg/m²) | 25.42 (±5.6) |
| Surgical admission | 132 (80%) |
| Abdominal surgery | 119 (72.1%) |
| PN started in the ICU | 71 (43%) |
| Hospitalization time (days) | 43 (27.5−64.5) |
| Charlson (comorbidity index) | 4 (2−6) |
| SAPS 3a | 63.4 (±14.5) |
| SOFAa | 5 (3−7) |
| Vasoactive drugsa | 21 (12.7%) |
| PN time (days) | 15 (9−25) |
| Total PN | 125 (75.7%) |
| Supplemental PN | 40 (24.2%) |
| Comorbidities | |
| DM | 35 (21.2%) |
| Coronary artery disease | 16 (9.7%) |
| Heart failure | 6 (3.6%) |
| Stroke | 16 (9.7%) |
| Pulmonary disease | 20 (12.1%) |
| Hepatic disease | 8 (4.8%) |
| Cancer | 73 (44.2%) |
| Chronic kidney disease | 13 (7.9%) |
| PN daily prescription | |
| Calories (kcal) | 1598 (±423.3) |
| Calories (kcal/kg) | 25.2 (20.2−27.6) |
| Protein (g/kg) | 1.5 (1.24−1.61) |
| Glucose (g/kg) | 3.08 (2.52−3.52) |
| Lipids (g/kg) | 0.8 (0.58−0.91) |
| Outcomes | |
| Mortality | 41 (24.8%) |
| Hyperglycemia | 62 (37.6%) |
| Any infection | 107 (64.8%) |
| Pulmonary infection | 28 (17%) |
| Abdominal infection | 60 (36.4%) |
| Operative wound infection | 7 (4.2%) |
| Urinary infection | 9 (5.5%) |
| Bacteremia not related to CVC | 7 (4.2%) |
| CLABSI | 24 (14.5%) |
| Fungemia | 12 (7.3%) |
N represents the number of patients (and percentage). Mean (± standard deviation) or median (interquartile range). BMI, body mass index; ICU, intensive care unit; SAPS 3, simplified acute physiology score 3; SOFA, sequential organ failure assessment; PN, parenteral nutrition; DM, diabetes mellitus; CVC, central venous catheter; and CLABSI, central line–associated bloodstream infection.
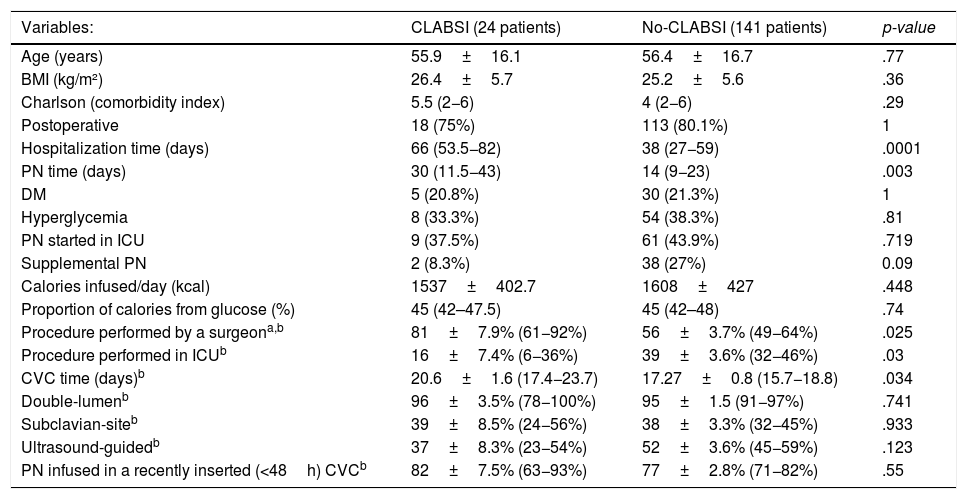
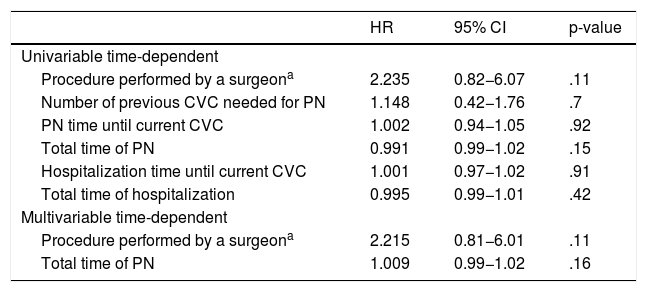
Table 2 summarizes the findings associated with CLABSI. There were 28 episodes of CLABSI (11.3% of 247 CVCs), but some events occurred in the same patient. At least one episode of bloodstream infection occurred in 24 patients (14.5% of 165 patients). Considering the time used for each CVC, the CLABSI index was 6.47 per 1000 CVC-days. In the univariable analysis, CLABSI was associated with longer hospitalization time, longer PN time, longer CVC time, catheter insertion performed by a surgeon or a surgical resident, and procedures performed outside the ICU. No association was found with total calories of PN, proportion of macronutrients, hyperglycemia, supplemental PN, use of ultrasound or comorbidities at the beginning of PN. Furthermore, no CLABSI occurred in less than 5 days of CVC use (median of 15 days), and using a recently inserted device (with less than 48h of use) when starting PN was not associated with a lower rate of CLABSI. In an extended time-dependent Cox regression, no variable was associated with a higher risk of CLABSI in the univariable and multivariable analysis (Table 3). Additional information about the 247 CVC insertion procedures is available in Supplementary Table.
Univariable analysis for evolution to CLABSI at hospitalization.
| Variables: | CLABSI (24 patients) | No-CLABSI (141 patients) | p-value |
|---|---|---|---|
| Age (years) | 55.9±16.1 | 56.4±16.7 | .77 |
| BMI (kg/m²) | 26.4±5.7 | 25.2±5.6 | .36 |
| Charlson (comorbidity index) | 5.5 (2−6) | 4 (2−6) | .29 |
| Postoperative | 18 (75%) | 113 (80.1%) | 1 |
| Hospitalization time (days) | 66 (53.5−82) | 38 (27−59) | .0001 |
| PN time (days) | 30 (11.5−43) | 14 (9−23) | .003 |
| DM | 5 (20.8%) | 30 (21.3%) | 1 |
| Hyperglycemia | 8 (33.3%) | 54 (38.3%) | .81 |
| PN started in ICU | 9 (37.5%) | 61 (43.9%) | .719 |
| Supplemental PN | 2 (8.3%) | 38 (27%) | 0.09 |
| Calories infused/day (kcal) | 1537±402.7 | 1608±427 | .448 |
| Proportion of calories from glucose (%) | 45 (42–47.5) | 45 (42–48) | .74 |
| Procedure performed by a surgeona,b | 81±7.9% (61−92%) | 56±3.7% (49−64%) | .025 |
| Procedure performed in ICUb | 16±7.4% (6−36%) | 39±3.6% (32−46%) | .03 |
| CVC time (days)b | 20.6±1.6 (17.4−23.7) | 17.27±0.8 (15.7−18.8) | .034 |
| Double-lumenb | 96±3.5% (78−100%) | 95±1.5 (91−97%) | .741 |
| Subclavian-siteb | 39±8.5% (24−56%) | 38±3.3% (32−45%) | .933 |
| Ultrasound-guidedb | 37±8.3% (23−54%) | 52±3.6% (45−59%) | .123 |
| PN infused in a recently inserted (<48h) CVCb | 82±7.5% (63−93%) | 77±2.8% (71−82%) | .55 |
BMI is body mass index; ICU, intensive care unit; PN, parenteral nutrition; DM, diabetes mellitus; CVC, central venous catheter; and CLABSI, central line–associated bloodstream infection. The cells represent N (%), mean±SD or median (interquartile range).
Evolution to CLABSI in a time-dependent Cox regression.
| HR | 95% CI | p-value | |
|---|---|---|---|
| Univariable time-dependent | |||
| Procedure performed by a surgeona | 2.235 | 0.82−6.07 | .11 |
| Number of previous CVC needed for PN | 1.148 | 0.42−1.76 | .7 |
| PN time until current CVC | 1.002 | 0.94−1.05 | .92 |
| Total time of PN | 0.991 | 0.99−1.02 | .15 |
| Hospitalization time until current CVC | 1.001 | 0.97−1.02 | .91 |
| Total time of hospitalization | 0.995 | 0.99−1.01 | .42 |
| Multivariable time-dependent | |||
| Procedure performed by a surgeona | 2.215 | 0.81−6.01 | .11 |
| Total time of PN | 1.009 | 0.99−1.02 | .16 |
Extended Cox model for time-dependent covariates, through "R" survival package. HR: Hazard Ratio; CI: 95% confidence interval. R square=0.019. Concordance=0.566. Likelihood ratio test=4.38. Wald test 4.28. Logrank test 4.54 p=0.1.
Regarding CLABSI epidemiology, coagulase-negative staphylococci were present in 13 cases (46.4%), followed by fungal infections (Candida) in eight cases (28.6%) and Staphylococcus aureus in two cases (7.1%). Klebsiella, Enterococcus, Pseudomonas, Enterobacter, and Escherichia were responsible for one case of CLABSI each (3.6%). The median time for blood culture positivity in CLABSI cases was 13.9h (12−24h) for peripheral blood cultures and 12.2h (9.9–19.8h) for blood cultures collected from the PN pathway.
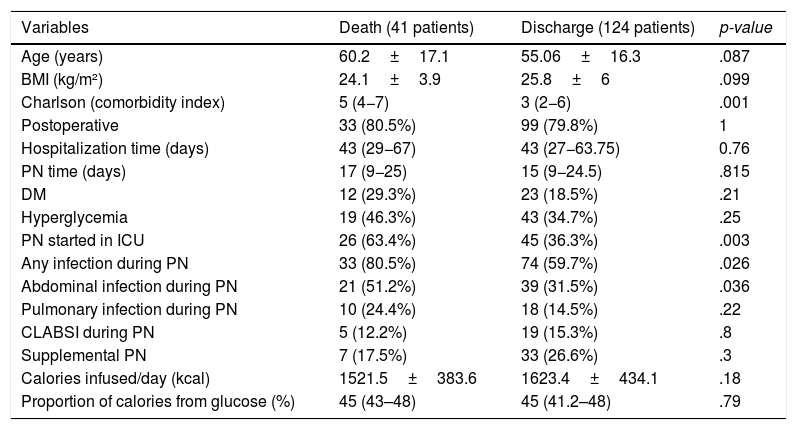
Overall mortality rate was 24.8% in this study. Higher Charlson index, starting PN in ICU, development of any infection during PN administration and development of abdominal infection during PN administration were related to death (Table 4). In multivariate analysis with these variables, only the Charlson comorbidity index remained significantly associated with mortality (HR 1.175; CI 1.052–1.312; p=.004).
Univariable analysis for evolution to death at hospitalization.
| Variables | Death (41 patients) | Discharge (124 patients) | p-value |
|---|---|---|---|
| Age (years) | 60.2±17.1 | 55.06±16.3 | .087 |
| BMI (kg/m²) | 24.1±3.9 | 25.8±6 | .099 |
| Charlson (comorbidity index) | 5 (4−7) | 3 (2−6) | .001 |
| Postoperative | 33 (80.5%) | 99 (79.8%) | 1 |
| Hospitalization time (days) | 43 (29−67) | 43 (27−63.75) | 0.76 |
| PN time (days) | 17 (9−25) | 15 (9−24.5) | .815 |
| DM | 12 (29.3%) | 23 (18.5%) | .21 |
| Hyperglycemia | 19 (46.3%) | 43 (34.7%) | .25 |
| PN started in ICU | 26 (63.4%) | 45 (36.3%) | .003 |
| Any infection during PN | 33 (80.5%) | 74 (59.7%) | .026 |
| Abdominal infection during PN | 21 (51.2%) | 39 (31.5%) | .036 |
| Pulmonary infection during PN | 10 (24.4%) | 18 (14.5%) | .22 |
| CLABSI during PN | 5 (12.2%) | 19 (15.3%) | .8 |
| Supplemental PN | 7 (17.5%) | 33 (26.6%) | .3 |
| Calories infused/day (kcal) | 1521.5±383.6 | 1623.4±434.1 | .18 |
| Proportion of calories from glucose (%) | 45 (43–48) | 45 (41.2–48) | .79 |
BMI represents body mass index; ICU, intensive care unit; PN, parenteral nutrition; DM, diabetes mellitus; CVC, central venous catheter; and CLABSI, central line–associated bloodstream infection. The cells represent N (%), mean±SD or median (interquartile range).
In this study, a large sample of patients submitted to PN over a two-year period in a university hospital of the South of Brazil was analyzed. To the best of our knowledge, this is one of the largest cohorts identified in the literature analyzing patients receiving PN both in the general ward and in the ICU settings. The rate of infectious complications in these individuals is high. Patients who needed PN had a higher incidence of CLABSI compared to patients with CVC and without PN in the literature,18,19 but no characteristics of PN studied were associated with CLABSI and additional days of PN did not increase the rate of CLABSI in multivariate analyses in our study.
In an earlier study conducted in the same hospital almost 20 years earlier,10 PN was shown to be independently associated with CLABSI in multivariate analysis. That study differs from the present investigation for including only ICU patients, and because microbiological analyses of all patients (blood culture or catheter tip) were performed. The association between PN and infection could be due to colonization of the device. Probably for the same reason, a two-fold higher rate of CLABSI per 1000 catheter days was identified in comparison with the current study, although improvements in procedures and in catheter care that have been established over time may have also influenced this difference.
The high incidence of CLABSI found in our study (6.47 per 1000 CVC-days or 11.7% of all CVCs) when compared to patients with CVC and without PN in the literature18–20 is still within the range (which reaches 18.8 per 1000 CVC-days) of the international literature for PN-associated CVC infection6). In a time-dependent Cox regression, PN time was not an independent factor that could justify a higher incidence of CLABSI in this population. It is difficult to identify reasons for this incidence, since the study was conducted in a university hospital accredited by the Joint Commission21 and specific bundles for CVC care are available in our hospital. Nevertheless, Brazil is an emerging country and data about catheter infection, especially in patients receiving PN, are scarce.
Dissanaike et al.22 found an association between CLABSI and higher total calorie infusion, which was not observed in our cohort. Most of the patients in Dissanaike’s study received more than 30−40kcal/kg/day, in contrast with the current study, where the local protocol encouraged a goal of 22−25kcal/kg/day and few patients received more than 30kcal/kg/day, in accordance with recent guidelines.3 We believe that such findings indicate that avoiding hyperalimentation may reduce the rate of CLABSI and other unfavorable outcomes, as already demonstrated by studies that limited total calories and compared parenteral and enteral nutrition using the same caloric target.4 One possible explanation for the high CLABSI rate in our study was the use of two-in-one bags separated from intravenous lipid emulsion that are supposed to be associated with an increased risk of infection, through CVC manipulation. However, this evidence is still limited and not sufficient to endorse or refute such an association.23
The present study did not identify lower rates of CLABSI when a new CVC was installed after indication of PN, thus not justifying the need for a new device or replacement of the CVC when initiating such therapy. Other catheter-related factors, such as the number of lumens, were also not associated with higher chance of infection in our study, although the analysis was not robust enough because of the low prevalence of mono-lumen catheters (less than 5%) used in our hospital. Therefore, it is impossible to refute this association found in the literature,24 and although recommended, there is a paucity of evidence regarding PN-dedicated lumens.25
Our mortality rate was high, and the age-adjusted Charlson comorbidity index indicates that our sample of patients was sicker than population in other PN studies, probably justifying the higher mortality.26,27 This comorbidity metrics is the most commonly studied prognostic measure of illness burden in clinical research28 and is probably related to increased rates of chronic disease and mortality.29–31 Our study failed to identify prolonged hospitalization or PN time as independent factors to justify this rate. Only greater number of comorbidities was associated with mortality in the multivariate analysis.
Among the limitations, the study methodology does not allow for cause-effect inference, although it is possible to generate hypotheses. Multivariate analysis and logistic regression were performed to mitigate bias and confounding. Furthermore, our high rate of infection does not invalidate the finding that CLABSI was not associated with specifics of PN or vascular access characteristics. In addition, the retrospective design may hinder outcome recovery and related factors due to underreporting in the medical records. To counteract underreporting, we chose laboratory results and the outcome of hospitalization (death or discharge) as the main outcomes. The study was not powered to detect mortality difference a priori, and this aspect should be considered when analyzing data.
The exclusion of LTC and PICC from the analyses is also a limitation. LTC may lead to underreporting due to their out-of-hospital use and possibility of lack of notification or even occurrence of an outcome in another institution. It has already been stated that PICC were used in the hospital during the study only in experimental situations and they were not analyzed because of the possibility of bias due to differentiated care involving a new technology. As a mitigating factor, less than 10 of these devices (seven LTC and two PICC) were used for PN in the hospital in the study period (3.6% of all catheters used for PN), possibly not affecting the results.
In conclusion, patients who needed PN in our study had a considerable rate of CLABSI and other infectious complications. No variable was associated with higher risk of CLABSI in the univariate and multivariate analyses after adjustment for catheter time. The mortality rate was high and not associated with PN in multivariate analyses, only with Charlson comorbidity index.
Declarations of interestNone.
We are grateful to PhD Vanessa Bielefeldt Leotti for assistance in statistical analysis. Support for the publication fee was provided by FIPE-HCPA (Fundação de Incentivo à Pesquisa e Eventos - Hospital de Clínicas de Porto Alegre).