The natural history of HIV infection has changed dramatically after the introduction of highly active antiretroviral therapy. Currently, opportunistic illnesses still represent a major cause of death and hospitalization in this population. In this study, we review the trends in opportunistic illnesses incidence rates and compare the results observed in high-income settings with that for low/middle-income settings, with special attention given to studies from Brazil.
MethodsWe systematically searched Pubmed, Web of Science, Lilacs and Google scholar for publications on HIV associated opportunistic illness. Studies reporting rates based on person-time for all opportunistic illnesses and/or the three opportunistic infections of interest, namely, Pneumocystis carinii pneumonia, cerebral toxoplasmosis, and Mycobacterium avium complex were included.
ResultsSignificant reductions in the incidence rates were demonstrated for opportunistic illnesses overall and also for the specific opportunistic infections included in the present study, both in high and low/middle-income settings. Out of the 37 studies included in the present review, almost 70% were from high-income settings. All the studies conducted in low/middle-income settings were single center studies and four were from Brazil. We found no study from Brazil reporting annual incidence rates of opportunistic illnesses.
ConclusionsOpportunistic illnesses remain an important public health problem. To better guide health policies in low/middle-income settings, multicenter cohort studies should be encouraged. Studies from Brazil are urgently needed to assess the current burden of opportunistic illnesses in our population and to support the planning of HIV/AIDS health care services organization.
The natural history of human immunodeficiency virus (HIV) infection and acquired immunodeficiency syndrome (AIDS) has changed dramatically since the onset of the epidemic in the 1980s. The landmark of this process was the introduction of highly active antiretroviral therapy (ART) in 1996. Despite the progress made in the treatment and control of HIV infection, HIV/AIDS persists as one of the main causes of death in the world, affecting individuals from both high-income and low-income settings.1 In addition, although an increase in non-AIDS associated morbidity and mortality has been observed, opportunistic infections remain a major cause of hospitalization and death in people living with HIV/AIDS in high and low-income settings.2–4
Currently, in the post-ART period, opportunistic illnesses are mainly related with late diagnosis and/or presentation to care, non-adherence to ART and HIV resistance to antiretroviral drugs.2,5 Late diagnosis and/or presentation to care is one of the most challenging aspects of the HIV epidemic. In Brazil, 34% of the patients still present with an opportunistic illness at the moment of ART initiation.6 Furthermore, non-adherence to ART results in virologic failure and disease progression. Factors associated with non-adherence, such as low educational level, young age, unemployment, alcoholism and use of illicit drugs represent an important socioeconomic problem, in particular for low/middle-income settings.7,8 Finally, multidrug resistance to antiretroviral drugs is a consequence of HIV exposure to ART, particularly in settings where non-adherence prevails.9
In this study, we review the trends in opportunistic illnesses incidence rates and compare the results observed in high-income settings (HIS) with that for low/middle-income settings (LMIS), with special attention given to studies from Brazil. We evaluate the impact ART has had in three specific opportunistic infections of particular importance to Brazil and contrast the patterns in the countries evaluated.
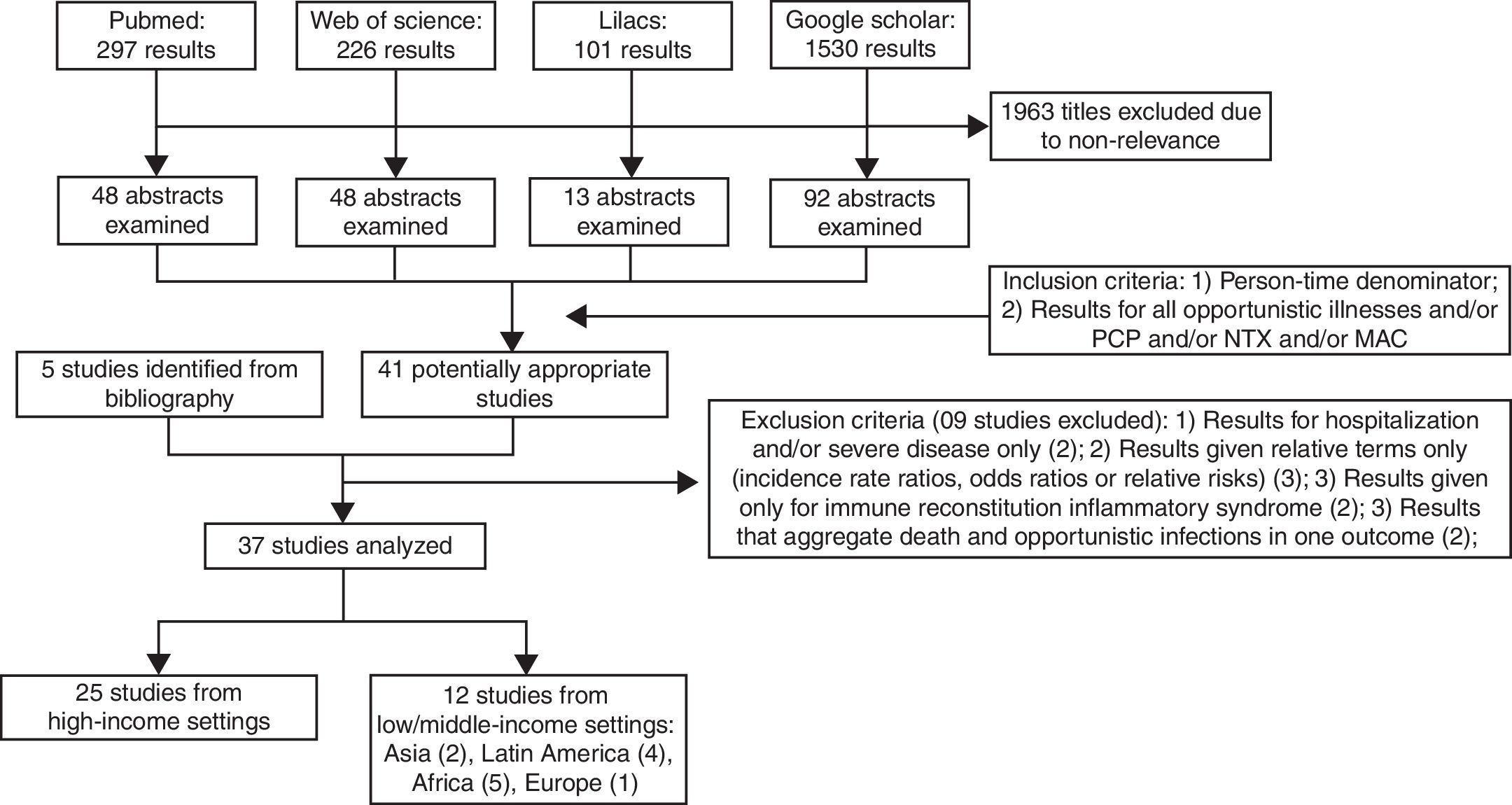
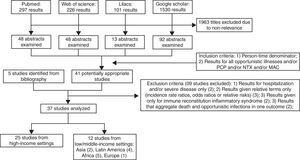
Search strategy and selection criteriaPublications related to AIDS-associated opportunistic illnesses incidence were identified by systematically searching in Pubmed, Web of Science, Lilacs and Google scholar. Publications were restricted to the following languages: English, Portuguese, and Spanish. The databases were searched for studies published until January 2013 using the following search terms and Boolean operators, for matches under any field: (incidence) AND (HIV OR human immunodeficiency virus) AND (AIDS-defining illness OR opportunistic infection OR opportunistic disease OR AIDS-related opportunistic infection OR AIDS-related opportunistic illness). For the Lilacs database, search terms were translated into Portuguese language and separate searches with each term were conducted. Titles and available abstracts were scanned for relevance identifying papers requiring further consideration. Bibliographies of relevant articles were also checked. Inclusion criteria consisted in (1) presence of a person-time denominator and (2) results for all opportunistic illnesses and/or the three opportunistic infections of interest, namely: Pneumocystis carinii pneumonia (PCP), cerebral toxoplasmosis (NTX) and Mycobacterium avium complex (MAC). Exclusion criteria included: (1) results given only for hospitalization and/or severe diseases, (2) results given relative terms only (that is, as incidence rate ratios, odds ratios or relative risks), (3) results given only for Immune Reconstitution Inflammatory Syndrome, and (4) results that aggregate death and opportunistic infections in one outcome. The results, inclusion and exclusion criteria are shown in Fig. 1.
ResultsThirty seven publications met the study's eligibility criteria, 25 from HIS and 12 studies from LMIS (Fig. 1).2,10–45 Out of the 12 studies from LMIS, four were from Latin America, specifically from Brazil. Results from these studies are summarized in the next sections with incidence rates in 100 person-years (100 PY) format.
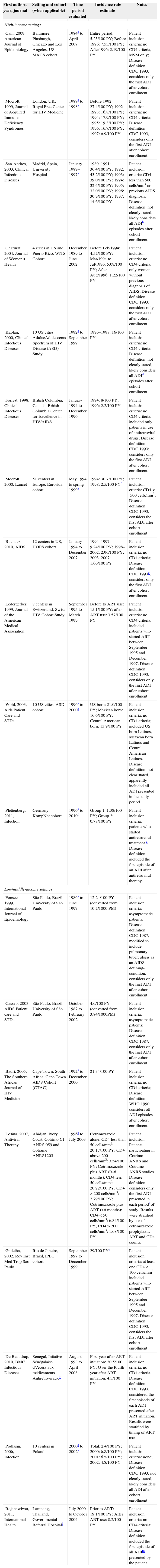
Opportunistic illnessesTable 1 summarizes the findings for the incidence rate of opportunistic illnesses from 1984 to 2010 in HIS and LMIS. Depending on the study, incidence rates ranged from 2.3 to 12.3 times lower in the post-ART period compared to the pre-ART period.
Incidence rates for opportunistic illnesses among HIV-infected individuals from high and low/middle-income settings.
| First author, year, journal | Setting and cohort (when applicable) | Time period evaluated | Incidence rate estimate | Notes |
|---|---|---|---|---|
| High-income settings | ||||
| Cain, 2009, American Journal of Epidemiology | Baltimore, Pittsburgh, Chicago and Los Angeles, US, MACS cohort | 1984a to April 2007 | Entire period: 5.23/100 PY; Before 1996: 7.53/100 PY; After1996: 2.19/100 PY | Patient inclusion criteria: no CD4 criteria, MSM only; Disease definition: CDC 1993, considers only the first ADI after cohort enrollment |
| Mocroft, 1999, Journal of Acquired Immune Deficiency Syndromes | London, UK, Royal Free Center for HIV Medicine | 1987a to 1998a | Before 1992: 27.4/100 PY; 1992–1993: 16.8/100 PY; 1994: 17.9/100 PY; 1995: 19.3/100 PY; 1996: 16.7/100 PY; 1997: 6.9/100 PY | Patient inclusion criteria: no CD4 criteria; Disease definition: CDC 1993, considers only the first ADI after cohort enrollment |
| San-Andres, 2003, Clinical Infectious Diseases | Madrid, Spain, University Hospital | January 1989–1997a | 1989–1991: 36.4/100 PY; 1992: 43.2/100 PY; 1993: 39.0/100 PY; 1994: 32.4/100 PY; 1995: 32.0/100 PY; 1996: 30.9/100 PY; 1997: 14.6/100 PY | Patient inclusion criteria: CD4 less than 500 cells/mm3 or previous AIDS diagnosis; Disease definition: not clearly stated, likely considers all ADIb episodes after cohort enrollment |
| Charurat, 2004, Journal of Women's Health | 4 states in US and Puerto Rico, WITS Cohort | December 1989 to June 2002 | Before Feb/1994: 4.52/100 PY; Mar/1994 to Jul/1996: 5.09/100 PY; After Aug/1996: 1.22/100 PY | Patient inclusion criteria: no CD4 criteria, only women without previous diagnosis of AIDS; Disease definition: CDC 1993; considers only the first ADI after cohort enrollment |
| Kaplan, 2000, Clinical Infectious Diseases | 10 US cities, Adults/Adolescents Spectrum of HIV Disease (ASD) Study | 1992a to September 1999 | 1996–1998: 16/100 PYc | Patient inclusion criteria: no CD4 criteria; Disease definition: not clearly stated, likely considers all ADId episodes after cohort enrollment |
| Forrest, 1998, Clinical Infectious Diseases | British Columbia, Canada, British Columbia Center for Excellence in HIV/AIDS | January 1994 to December 1996 | 1994: 8/100 PY; 1996: 2.2/100 PY | Patient inclusion criteria: no CD4 criteria, included only patients in use of antiretroviral drugs; Disease definition: CDC 1993; considers only the first ADI after cohort enrollment |
| Mocroft, 2000, Lancet | 51 centers in Europe, Eurosida cohort | May 1994 to spring 1999a | 1994: 30.7/100 PY; 1998: 2.5/100 PYc | Patient inclusion criteria: CD4<500 cells/mm3; Disease definition: CDC 1993, considers the first ADI after cohort enrollment |
| Buchacz, 2010, AIDS | 12 centers in US, HOPS cohort | January 1994 to December 2007 | 1994–1997: 9.24/100 PY; 1998–2002: 2.96/100 PY; 2003–2007: 1.66/100 PY | Patient inclusion criteria: no CD4 criteria; Disease definition: CDC 1993e; considers only the first ADI after cohort enrollment |
| Ledergerber, 1999, Journal of the American Medical Association | 7 centers in Switzerland, Swiss HIV Cohort Study | September 1995 to March 1999 | Before to ART use: 15.1/100 PY; after ART use: 3.57/100 PY | Patient inclusion criteria: no CD4 criteria, included patients who started ART between September 1995 and December 1997. Disease definition: CDC 1993, considers only the first ADI after cohort enrollment |
| Wohl, 2003, Aids Patient Care and STDs | 10 US cities, ASD cohort | 1996a to 2000a | US born: 21.0/100 PY; Mexican born: 16.6/100 PY; Central American born: 13.9/100 PY | Patient inclusion criteria: no CD4 criteria; included US born Latinos, Mexican born Latinos and Central American Latinos. Disease definition: not clear stated, apparently included all ADI presented in the study period. |
| Plettenberg, 2011, Infection | Germany, KompNet cohort | 1996a to 2010f | Group 1: 1.38/100 PY; Group 2: 0.78/100 PY | Patient inclusion criteria: patients who started antiretroviral treatment.g Disease definition: included the first episode of an ADI after antiretroviral therapy. |
| Low/middle-income settings | ||||
| Fonseca, 1999, International Journal of Epidemiology | São Paulo, Brazil, University of São Paulo | 1986a to June 1997 | 12.24/100 PY (converted from 10.2/1000 PM) | Patient inclusion criteria: asymptomatic patients; Disease definition: CDC 1987, modified to include pulmonary tuberculosis as an AIDS defining-condition, considers only the first ADI after cohort enrollment |
| Casseb, 2003, AIDS Patient care and STDs | São Paulo, Brazil, University of São Paulo | October 1987 to February 2002 | 4.6/100 PY (converted from 3.84/1000PM) | Patient inclusion criteria: asymptomatic patients; Disease definition: CDC 1987, considers only the first ADI after cohort enrollment |
| Badri, 2005, The Southern African Journal of HIV Medicine | Cape Town, South Africa, Cape Town AIDS Cohort (CTAC) | 1992a to December 2000 | 21.34/100 PY | Patient inclusion criteria: no CD4 criteria; Disease definition: WHO 1990, considers all ADI episodes after cohort enrollment |
| Losina, 2007, Antiviral Therapy | Abidjan, Ivory Coast, Cotrimo CI ANRS 059 and Cotrame ANRS1203 | 1996a to July 2003 | Cotrimoxazole alone: CD4 less than 50 cells/mm3: 20.17/100 PY; CD4 above 200 cells/mm3: 3.54/100 PY; Cotrimoxazole plus ART (0–6 months): CD4 less 50 cells/mm3: 20.22/100 PY, CD4>200 cells/mm3: 2.79/100 PY; Cotrimoxazole plus ART (>6 months): CD4<50 cells/mm3: 6.84/100 PY, CD4>200 cells/mm3: 1.68/100 PY | Patient inclusion: Patients participating in Cotrimo ANRS and Cotrame ANRS studies. Disease definition: considers only the first ADIh presented in each periodi of study. Results were stratified by use of cotrimoxazole prophylaxis, ART and CD4 counts. |
| Gadelha, 2002, Rev Inst Med Trop Sao Paulo | Rio de Janeiro, Brazil, IPEC cohort | September 1997 to December 1999 | 29/100 PYj | Patient inclusion criteria: at least one CD4<100 cells/mm3, included patients who started ART between September 1995 and December 1997. Disease definition: CDC 1993, considers the first ADI after cohort enrollment |
| De Beaudrap, 2010, BMC Infectious Diseases | Senegal, Initative Sénégalaise d’Acèss aux médicaments Antiretrovirauxk | August 1998 to April 2008 | First year after ART initiation: 20.5/100 PY. Over the fourth year after ART initiation: 4.3/100 PY | Patient inclusion criteria: no CD4 criteria. Disease definition: CDC 1993, considered the first episode of each ADI presented after ART initiation. Results were stratified by timing of ART use |
| Podlasin, 2006, Infection | 10 centers in Poland | 2000a to 2002a | Total: 2.4/100 PY; 2000: 6.8/100 PY; 2001: 6.5/100 PY; 2002: 4.8/100 PY | Patient inclusion criteria: none; Disease definition: CDC 1993, not clearly stated, likely considers all ADI after cohort enrollment |
| Rojanawiwat, 2011, International Health | Lampang, Thailand, Governmental Referral Hospitall | July 2000 to October 2004 | Prior to ART: 19.1/100 PY; After ART use: 8.2/100 PY | Patient inclusion criteria: no CD4 criteria; Disease definition: included the first episode of all ADIm presented by the patient |
ADI: AIDS defining illness; CDC: Centers for Disease Control; CMV: cytomegalovirus; MAC: Mycobacterium avium complex; MSM: men who have sex with men; PCP: Pneumocystis carinii pneumonia.
Does not specify the criteria used for ADI, the results include: Esophageal candidiasis, PCP, tuberculosis, wasting syndrome, cerebral toxoplasmosis, Kaposi's sarcoma, AIDS dementia complex, progressive multifocal leukoencephalopathy, primary brain lymphoma, CMV disease, MAC, non-Hodgkin lymphoma, cryptosporidiosis, recurrent pneumonia, cryptococcosis, chronic herpes simplex, invasive cervical cancer.
Diseases included: PCP, disseminated MAC, cerebral toxoplasmosis, Kaposi's sarcoma, CMV retinitis, esophageal candidiasis, cryptococcosis.
Patients were separated into two groups: Group 1: patients who started ART with CD4 between 250 and 349 cells/mm3; Group 2: patients who started ART with CD4 between 350 and 450 cells/mm3.
Diseases included: severe bacterial infections (pneumonia, enteritis, bacteremia, invasive urogenital infection), malaria, cerebral toxoplasmosis, isosporosis, PCP, extrapulmonar cryptococosis, esophageal candidiasis, tuberculosis, MAC, other WHO clinical stage 3 and 4.
In the first period (until December 1998), patients received cotrimoxazole prophylaxis. In the second period (after December 1998) patients received ART plus cotrimoxazole prophylaxis (the later period was separated in the first 6 months after ART initiation and after 6 months of ART initiation).
In HIS, a multicenter study conducted in the United States using data from the HIV Outpatient Study (HOPS) cohort with no CD4+ cell count restriction of the study population reported that the incidence rate of opportunistic illnesses decreased from 9.24/100 PY in pre-ART period to 1.66/100 PY in post-ART period.2 A more striking result was reported for the Eurosida cohort, an European multicenter cohort that included only patients with CD4+ cell counts less than 500 cells/mm3 where the incidence rate of opportunistic illnesses decreased from 30.7/100 PY in the pre-ART period to 2.5/100 PY in the post-ART period.10 Similarly, a study from Spain that included patients with CD4+ cell counts less than 500 cells/mm3 reported significant decreases in the incidence rate of opportunistic illnesses, which went from 43.2/100 PY to 14.6/100 PY, in the pre and post-ART periods, respectively.11 Other studies conducted in HIS can be found in Table 1, including results from England, Canada, Switzerland and Germany.
In LMIS, in a study from Thailand with no CD4+ cell count restriction of the study population, the incidence rate of opportunistic illnesses decreased from 19.1/100 PY in the absence of ART to 8.2/100 PY after ART use.12 A study conducted in São Paulo in the period of 1986 through 1997, also with no CD4+ cell count restriction of the study population, reported an incidence rate of opportunistic illnesses of 12.24/100 PY in a supposedly pre-ART period.13 Another study conducted with the same population during the period from 1987 to 2002 estimated a lower incidence rate of opportunistic illnesses of 4.6/100 PY.14 A study from Rio de Janeiro, that included only patients with CD4+ counts less than 100 cells/mm3 in the period of 1997 to 1999, found an incidence rate of opportunistic illnesses of 29/100 PY a supposedly post-ART period.15 Other studies conducted in LMIS can be found in Table 1 and include results from South Africa, Ivory Coast, Senegal and Poland.
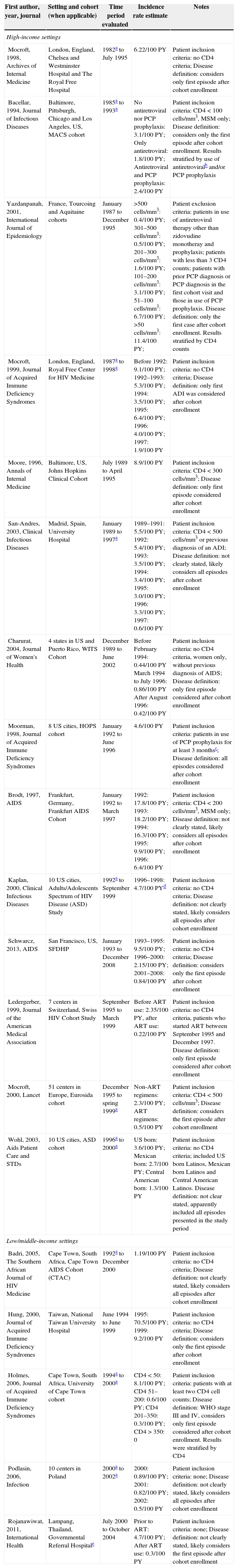
Pneumocystis carinii pneumoniaTable 2 summarizes the findings for PCP incidence rates from 1982 to 2008 in HIS and LMIS. Depending on the study, incidence rates ranged from 2.0 to 15.6 times lower in the post-ART period compared to the pre-ART period.
Incidence rate for Pneumocystis carinii pneumonia among HIV-infected individuals from high and low/middle-income settings.
| First author, year, journal | Setting and cohort (when applicable) | Time period evaluated | Incidence rate estimate | Notes |
|---|---|---|---|---|
| High-income settings | ||||
| Mocroft, 1998, Archives of Internal Medicine | London, England, Chelsea and Westminster Hospital and The Royal Free Hospital | 1982a to July 1995 | 6.22/100 PY | Patient inclusion criteria: no CD4 criteria; Disease definition: considers only first episode after cohort enrollment |
| Bacellar, 1994, Journal of Infectious Diseases | Baltimore, Pittsburgh, Chicago and Los Angeles, US, MACS cohort | 1985a to 1993a | No antiretroviral nor PCP prophylaxis: 3.1/100 PY; Only antiretroviral: 1.8/100 PY; Antiretroviral and PCP prophylaxis: 2.4/100 PY | Patient inclusion criteria: CD4<100 cells/mm3, MSM only; Disease definition: considers only the first episode after cohort enrollment. Results stratified by use of antiretroviralb and/or PCP prophylaxis |
| Yazdanpanah, 2001, International Journal of Epidemiology | France, Tourcoing and Aquitaine cohorts | January 1987 to December 1995 | >500 cells/mm3: 0.4/100 PY; 301–500 cells/mm3: 0.5/100 PY; 201–300 cells/mm3: 1.6/100 PY; 101–200 cells/mm3: 3.1/100 PY; 51–100 cells/mm3: 6.7/100 PY; >50 cells/mm3: 11.4/100 PY; | Patient exclusion criteria: patients in use of antiretroviral therapy other than zidovudine monotheray and prophylaxis; patients with less than 3 CD4 counts; patients with prior PCP diagnosis or PCP diagnosis in the first cohort visit and those in use of PCP prophylaxis. Disease definition: only the first case after cohort enrollment. Results stratified by CD4 counts |
| Mocroft, 1999, Journal of Acquired Immune Deficiency Syndromes | London, England, Royal Free Center for HIV Medicine | 1987a to 1998a | Before 1992: 9.1/100 PY; 1992–1993: 5.3/100 PY; 1994: 3.5/100 PY; 1995: 6.4/100 PY; 1996: 4.0/100 PY; 1997: 1.9/100 PY | Patient inclusion criteria: no CD4 criteria; Disease definition: only first ADI was considered after cohort enrollment |
| Moore, 1996, Annals of Internal Medicine | Baltimore, US, Johns Hopkins Clinical Cohort | July 1989 to April 1995 | 8.9/100 PY | Patient inclusion criteria: CD4<300 cells/mm3; Disease definition: only first episode considered after cohort enrollment |
| San-Andres, 2003, Clinical Infectious Diseases | Madrid, Spain, University Hospital | January 1989 to 1997a | 1989–1991: 5.5/100 PY; 1992: 5.4/100 PY; 1993: 3.5/100 PY; 1994: 3.4/100 PY; 1995: 3.0/100 PY; 1996: 3.3/100 PY; 1997: 0.6/100 PY | Patient inclusion criteria: CD4<500 cells/mm3 or previous diagnosis of an ADI; Disease definition: not clearly stated, likely considers all episodes after cohort enrollment |
| Charurat, 2004, Journal of Women's Health | 4 states in US and Puerto Rico, WITS Cohort | December 1989 to June 2002 | Before February 1994: 0.44/100 PY March 1994 to July 1996: 0.86/100 PY After August 1996: 0.42/100 PY | Patient inclusion criteria: no CD4 criteria, women only, without previous diagnosis of AIDS; Disease definition: only first episode considered after cohort enrollment |
| Moorman, 1998, Journal of Acquired Immune Deficiency Syndromes | 8 US cities, HOPS cohort | January 1992 to June 1996 | 4.6/100 PY | Patient inclusion criteria: patients in use of PCP prophylaxis for at least 3 monthsc; Disease definition: all episodes considered after cohort enrollment |
| Brodt, 1997, AIDS | Frankfurt, Germany, Frankfurt AIDS Cohort | January 1992 to March 1997 | 1992: 17.8/100 PY; 1993: 18.2/100 PY; 1994: 16.3/100 PY; 1995: 9.9/100 PY; 1996: 6.4/100 PY | Patient inclusion criteria: CD4<200 cells/mm3, MSM only; Disease definition: not clearly stated, likely considers all episodes after cohort enrollment |
| Kaplan, 2000, Clinical Infectious Diseases | 10 US cities, Adults/Adolescents Spectrum of HIV Disease (ASD) Study | 1992a to September 1999 | 1996–1998: 4.7/100 PYd | Patient inclusion criteria: no CD4 criteria; Disease definition: not clearly stated, likely considers all episodes after cohort enrollment |
| Schwarcz, 2013, AIDS | San Francisco, US, SFDHP | January 1993 to December 2008 | 1993–1995: 9.5/100 PY; 1996–2000: 2.15/100 PY; 2001–2008: 0.84/100 PY | Patient inclusion criteria: no CD4 criteria; Disease definition: considers only the first episode after cohort enrollment |
| Ledergerber, 1999, Journal of the American Medical Association | 7 centers in Switzerland, Swiss HIV Cohort Study | September 1995 to March 1999 | Before ART use: 2.35/100 PY, after ART use: 0.22/100 PY | Patient inclusion criteria: no CD4 criteria, patients who started ART between September 1995 and December 1997. Disease definition: only first episode considered after cohort enrollment |
| Mocroft, 2000, Lancet | 51 centers in Europe, Eurosida cohort | December 1995 to spring 1999a | Non-ART regimens: 2.3/100 PY; ART regimens: 0.5/100 PY | Patient inclusion criteria: CD4<500 cells/mm3; Disease definition: considers the first episode after cohort enrollment |
| Wohl, 2003, Aids Patient Care and STDs | 10 US cities, ASD cohort | 1996a to 2000a | US born: 3.6/100 PY; Mexican born: 2.7/100 PY; Central American born: 1.3/100 PY | Patient inclusion criteria: no CD4 criteria; included US born Latinos, Mexican born Latinos and Central American Latinos. Disease definition: not clear stated, apparently included all episodes presented in the study period |
| Low/middle-income settings | ||||
| Badri, 2005, The Southern African Journal of HIV Medicine | Cape Town, South Africa, Cape Town AIDS Cohort (CTAC) | 1992a to December 2000 | 1.19/100 PY | Patient inclusion criteria: no CD4 criteria; Disease definition: not clearly stated, likely considers all episodes after cohort enrollment |
| Hung, 2000, Journal of Acquired Immune Deficiency Syndromes | Taiwan, National Taiwan University Hospital | June 1994 to June 1999 | 1995: 70.5/100 PY; 1999: 9.2/100 PY | Patient inclusion criteria: no CD4 criteria; Disease definition: considers only the first episode after cohort enrollment |
| Holmes, 2006, Journal of Acquired Immune Deficiency Syndromes | Cape Town, South Africa, University of Cape Town cohort | 1994a to 2000a | CD4<50: 8.1/100 PY; CD4 51–200: 0.6/100 PY; CD4 201–350: 0.3/100 PY; CD4>350: 0 | Patient inclusion criteria: patients with at least two CD4 cell counts; Disease definition: WHO stage III and IV, considers only first episode considered after cohort enrollment. Results were stratified by CD4 |
| Podlasin, 2006, Infection | 10 centers in Poland | 2000a to 2002a | 2000: 0.89/100 PY; 2001: 0.82/100 PY; 2002: 0.5/100 PY | Patient inclusion criteria: none; Disease definition: not clearly stated, likely considers all episodes after cohort enrollment |
| Rojanawiwat, 2011, International Health | Lampang, Thailand, Governmental Referral Hospitale | July 2000 to October 2004 | Prior to ART: 4.7/100 PY; After ART use: 0.3/100 PY | Patient inclusion criteria: none; Disease definition: not clearly stated, likely considers the first episode after cohort enrollment |
ADI: AIDS defining illness; ART: highly active antiretroviral therapy; MSM: men who have sex with men; PCP: Pneumocytis carinii pneumonia.
In HIS, a study conducted in one center in England including all HIV-infected individuals showed that the incidence rate of PCP decreased from 9.1/100 PY in the pre-ART period (before 1992) to 1.9/100 PY in the post-ART period (in 1997).16 An even more dramatic result was reported in a study from San Francisco, United States, that used local surveillance data of the HIV-infected population and showed that the incidence rate of PCP dropped from 9.5/100 PY in pre-ART period (1993–1995) to 0.85/100 PY in post-ART period (2001–2008).17 Other studies conducted in HIS can be found in Table 2, including results from France, Spain, Switzerland and Germany.
In LMIS, a study from Taiwan that included all HIV-infected individuals estimated that the incidence rate of PCP decreased from 70.5/100 PY in the pre-ART period (1995) to 9.2/100 PY in the post-ART period (1999).18 In addition, a study from Thailand that, again, included all HIV-infected patients reported an incidence rate of PCP decreasing from 4.7/100 PY in the absence of ART to 0.3/100 PY after ART use.12 Other studies conducted in LMIS can be found in Table 2 and include results from South Africa and Poland. Unfortunately, we found no study from Brazil.
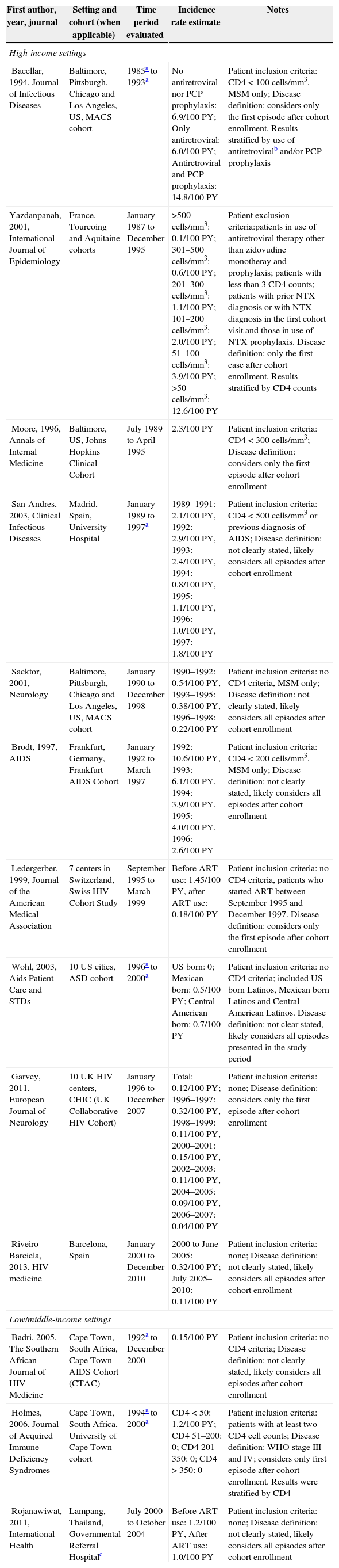
Cerebral toxoplasmosisTable 3 summarizes the findings for NXT incidence rate from 1985 to 2010 in HIS and LMIS. Depending on the study, incidence rates varied from 1.2 to 8.0 times lower in the post-ART period compared to the pre-ART period.
Incidence rates for cerebral toxoplasmosis among HIV-infected individuals from high and low/middle-income settings.
| First author, year, journal | Setting and cohort (when applicable) | Time period evaluated | Incidence rate estimate | Notes |
|---|---|---|---|---|
| High-income settings | ||||
| Bacellar, 1994, Journal of Infectious Diseases | Baltimore, Pittsburgh, Chicago and Los Angeles, US, MACS cohort | 1985a to 1993a | No antiretroviral nor PCP prophylaxis: 6.9/100 PY; Only antiretroviral: 6.0/100 PY; Antiretroviral and PCP prophylaxis: 14.8/100 PY | Patient inclusion criteria: CD4<100 cells/mm3, MSM only; Disease definition: considers only the first episode after cohort enrollment. Results stratified by use of antiretroviralb and/or PCP prophylaxis |
| Yazdanpanah, 2001, International Journal of Epidemiology | France, Tourcoing and Aquitaine cohorts | January 1987 to December 1995 | >500 cells/mm3: 0.1/100 PY; 301–500 cells/mm3: 0.6/100 PY; 201–300 cells/mm3: 1.1/100 PY; 101–200 cells/mm3: 2.0/100 PY; 51–100 cells/mm3: 3.9/100 PY; >50 cells/mm3: 12.6/100 PY | Patient exclusion criteria:patients in use of antiretroviral therapy other than zidovudine monotheray and prophylaxis; patients with less than 3 CD4 counts; patients with prior NTX diagnosis or with NTX diagnosis in the first cohort visit and those in use of NTX prophylaxis. Disease definition: only the first case after cohort enrollment. Results stratified by CD4 counts |
| Moore, 1996, Annals of Internal Medicine | Baltimore, US, Johns Hopkins Clinical Cohort | July 1989 to April 1995 | 2.3/100 PY | Patient inclusion criteria: CD4<300 cells/mm3; Disease definition: considers only the first episode after cohort enrollment |
| San-Andres, 2003, Clinical Infectious Diseases | Madrid, Spain, University Hospital | January 1989 to 1997a | 1989–1991: 2.1/100 PY, 1992: 2.9/100 PY, 1993: 2.4/100 PY, 1994: 0.8/100 PY, 1995: 1.1/100 PY, 1996: 1.0/100 PY, 1997: 1.8/100 PY | Patient inclusion criteria: CD4<500 cells/mm3 or previous diagnosis of AIDS; Disease definition: not clearly stated, likely considers all episodes after cohort enrollment |
| Sacktor, 2001, Neurology | Baltimore, Pittsburgh, Chicago and Los Angeles, US, MACS cohort | January 1990 to December 1998 | 1990–1992: 0.54/100 PY, 1993–1995: 0.38/100 PY, 1996–1998: 0.22/100 PY | Patient inclusion criteria: no CD4 criteria, MSM only; Disease definition: not clearly stated, likely considers all episodes after cohort enrollment |
| Brodt, 1997, AIDS | Frankfurt, Germany, Frankfurt AIDS Cohort | January 1992 to March 1997 | 1992: 10.6/100 PY, 1993: 6.1/100 PY, 1994: 3.9/100 PY, 1995: 4.0/100 PY, 1996: 2.6/100 PY | Patient inclusion criteria: CD4<200 cells/mm3, MSM only; Disease definition: not clearly stated, likely considers all episodes after cohort enrollment |
| Ledergerber, 1999, Journal of the American Medical Association | 7 centers in Switzerland, Swiss HIV Cohort Study | September 1995 to March 1999 | Before ART use: 1.45/100 PY, after ART use: 0.18/100 PY | Patient inclusion criteria: no CD4 criteria, patients who started ART between September 1995 and December 1997. Disease definition: considers only the first episode after cohort enrollment |
| Wohl, 2003, Aids Patient Care and STDs | 10 US cities, ASD cohort | 1996a to 2000a | US born: 0; Mexican born: 0.5/100 PY; Central American born: 0.7/100 PY | Patient inclusion criteria: no CD4 criteria; included US born Latinos, Mexican born Latinos and Central American Latinos. Disease definition: not clear stated, likely considers all episodes presented in the study period |
| Garvey, 2011, European Journal of Neurology | 10 UK HIV centers, CHIC (UK Collaborative HIV Cohort) | January 1996 to December 2007 | Total: 0.12/100 PY; 1996–1997: 0.32/100 PY, 1998–1999: 0.11/100 PY, 2000–2001: 0.15/100 PY, 2002–2003: 0.11/100 PY, 2004–2005: 0.09/100 PY, 2006–2007: 0.04/100 PY | Patient inclusion criteria: none; Disease definition: considers only the first episode after cohort enrollment |
| Riveiro-Barciela, 2013, HIV medicine | Barcelona, Spain | January 2000 to December 2010 | 2000 to June 2005: 0.32/100 PY; July 2005–2010: 0.11/100 PY | Patient inclusion criteria: none; Disease definition: not clearly stated, likely considers all episodes after cohort enrollment |
| Low/middle-income settings | ||||
| Badri, 2005, The Southern African Journal of HIV Medicine | Cape Town, South Africa, Cape Town AIDS Cohort (CTAC) | 1992a to December 2000 | 0.15/100 PY | Patient inclusion criteria: no CD4 criteria; Disease definition: not clearly stated, likely considers all episodes after cohort enrollment |
| Holmes, 2006, Journal of Acquired Immune Deficiency Syndromes | Cape Town, South Africa, University of Cape Town cohort | 1994a to 2000a | CD4<50: 1.2/100 PY; CD4 51–200: 0; CD4 201–350: 0; CD4>350: 0 | Patient inclusion criteria: patients with at least two CD4 cell counts; Disease definition: WHO stage III and IV; considers only first episode after cohort enrollment. Results were stratified by CD4 |
| Rojanawiwat, 2011, International Health | Lampang, Thailand, Governmental Referral Hospitalc | July 2000 to October 2004 | Before ART use: 1.2/100 PY, After ART use: 1.0/100 PY | Patient inclusion criteria: none; Disease definition: not clearly stated, likely considers all episodes after cohort enrollment |
ART: highly active antiretroviral therapy; MSM: men who have sex with men; NTX: Cerebral toxoplasmosis; PCP: Pneumocytis carinii pneumonia.
In HIS, a multicenter cohort (Multicenter AIDS Cohort Study – MACS) of HIV-infected men who have sex with men from the United States reported that the incidence rate of NTX decreased from 0.54/100 PY in pre-ART period (1990–1992) to 0.22/100 PY in post-ART period (1996–1998).19 Data of the Swiss cohort (multicenter cohort) confirmed this trend showing that the incidence rate of NTX among HIV-infected individuals who started antiretroviral therapy between 1995 and 1997 decreased from 1.45/100 PY before ART use to 0.18/100 PY after ART use.20 Also in HIS, in a multicenter study from United Kingdom conducted among HIV-infected individuals reported that the incidence rate of NTX decreased from 0.32/100 PY in the pre-ART period (1996–1997) to 0.04/100 PY in the post-ART period (1996–2007).21 Other studies conducted in HIS can be found in Table 3, including results from England, France, Spain, Switzerland and Germany.
In LMIS, a study from Thailand that included all HIV-infected patients estimated a reduction in the incidence rate of NTX from 1.2/100 PY in the absence of ART to 1.0/100 PY after ART use.12 Data from LMIS also include a study from South Africa, with no CD4+ cell count restriction of the study population, with an incidence rate of NTX of 0.15/100 PY in the period of 1992–2000.22 Again, we unfortunately did not find any study from Brazil.
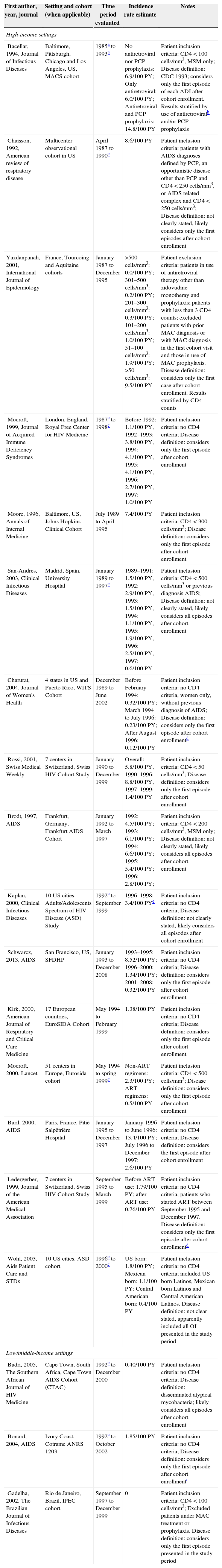
Mycobacterium avium complex diseaseTable 4 summarizes the findings for MAC incidence rate from 1985 to 2008 in HIS and LMIS. Depending on the study, incidence rates ranged from 2.4 to 25.8 times lower in the post-ART period compared to the pre-ART period.
Incidence rate of Mycobacterium avium complex among HIV-infected individuals from high and low/middle-income settings.
| First author, year, journal | Setting and cohort (when applicable) | Time period evaluated | Incidence rate estimate | Notes |
|---|---|---|---|---|
| High-income settings | ||||
| Bacellar, 1994, Journal of Infectious Diseases | Baltimore, Pittsburgh, Chicago and Los Angeles, US, MACS cohort | 1985a to 1993a | No antiretroviral nor PCP prophylaxis: 6.9/100 PY; Only antiretroviral: 6.0/100 PY; Antiretroviral and PCP prophylaxis: 14.8/100 PY | Patient inclusion criteria: CD4<100 cells/mm3, MSM only; Disease definition: CDC 1993; considers only the first episode of each ADI after cohort enrollment. Results stratified by use of antiretroviralb and/or PCP prophylaxis |
| Chaisson, 1992, American review of respiratory disease | Multicenter observational cohort in US | April 1987 to 1990c | 8.6/100 PY | Patient inclusion criteria: patients with AIDS diagnoses defined by PCP, an opportunistic disease other than PCP and CD4<250 cells/mm3, or AIDS related complex and CD4<250 cells/mm3; Disease definition: not clearly stated, likely considers only the first episodes after cohort enrollment |
| Yazdanpanah, 2001, International Journal of Epidemiology | France, Tourcoing and Aquitaine cohorts | January 1987 to December 1995 | >500 cells/mm3: 0.0/100 PY; 301–500 cells/mm3: 0.2/100 PY; 201–300 cells/mm3: 0.3/100 PY; 101–200 cells/mm3: 1.0/100 PY; 51–100 cells/mm3: 1.9/100 PY; >50 cells/mm3: 9.5/100 PY | Patient exclusion criteria: patients in use of antiretroviral therapy other than zidovudine monotheray and prophylaxis; patients with less than 3 CD4 counts; excluded patients with prior MAC diagnosis or with MAC diagnosis in the first cohort visit and those in use of MAC prophylaxis. Disease definition: considers only the first case after cohort enrollment. Results stratified by CD4 counts |
| Mocroft, 1999, Journal of Acquired Immune Deficiency Syndromes | London, England, Royal Free Center for HIV Medicine | 1987c to 1998c | Before 1992: 1.1/100 PY, 1992–1993: 3.8/100 PY, 1994: 4.1/100 PY, 1995: 4.1/100 PY, 1996: 2.7/100 PY, 1997: 1.0/100 PY | Patient inclusion criteria: no CD4 criteria; Disease definition: considers only the first episode after cohort enrollment |
| Moore, 1996, Annals of Internal Medicine | Baltimore, US, Johns Hopkins Clinical Cohort | July 1989 to April 1995 | 7.4/100 PY | Patient inclusion criteria: CD4<300 cells/mm3; Disease definition: considers only the first episode after cohort enrollment |
| San-Andres, 2003, Clinical Infectious Diseases | Madrid, Spain, University Hospital | January 1989 to 1997c | 1989–1991: 1.5/100 PY, 1992: 2.9/100 PY, 1993: 1.5/100 PY, 1994: 1.1/100 PY, 1995: 1.9/100 PY, 1996: 2.5/100 PY, 1997: 0.6/100 PY | Patient inclusion criteria: CD4<500 cells/mm3 or previous diagnosis AIDS; Disease definition: not clearly stated, likely considers all episodes after cohort enrollment |
| Charurat, 2004, Journal of Women's Health | 4 states in US and Puerto Rico, WITS Cohort | December 1989 to June 2002 | Before February 1994: 0.32/100 PY; March 1994 to July 1996: 0.23/100 PY; After August 1996: 0.12/100 PY | Patient inclusion criteria: no CD4 criteria, women only, without previous diagnosis of AIDS; Disease definition: considers only the first episode after cohort enrollmentd |
| Rossi, 2001, Swiss Medical Weekly | 7 centers in Switzerland, Swiss HIV Cohort Study | January 1990 to December 1999 | Overall: 5.8/100 PY, 1990–1996: 8.8/100 PY, 1997–1999: 1.4/100 PY | Patient inclusion criteria: CD4<50 cells/mm3; Disease definition: considers only the first episode after cohort enrollment |
| Brodt, 1997, AIDS | Frankfurt, Germany, Frankfurt AIDS Cohort | January 1992 to March 1997 | 1992: 4.5/100 PY; 1993: 6.1/100 PY; 1994: 6.6/100 PY; 1995: 5.4/100 PY; 1996: 2.8/100 PY; | Patient inclusion criteria: CD4<200 cells/mm3, MSM only; Disease definition: not clearly stated, likely considers all episodes after cohort enrollment |
| Kaplan, 2000, Clinical Infectious Diseases | 10 US cities, Adults/Adolescents Spectrum of HIV Disease (ASD) Study | 1992c to September 1999 | 1996–1998: 3.4/100 PYe | Patient inclusion criteria: no CD4 criteria; Disease definition: not clearly stated, likely considers all episodes after cohort enrollment |
| Schwarcz, 2013, AIDS | San Francisco, US, SFDHP | January 1993 to December 2008 | 1993–1995: 8.52/100 PY; 1996–2000: 1.34/100 PY; 2001–2008: 0.32/100 PY | Patient inclusion criteria: no CD4 criteria; Disease definition: considers only the first episode after cohort enrollment |
| Kirk, 2000, American Journal of Respiratory and Critical Care Medicine | 17 European countries, EuroSIDA Cohort | May 1994 to February 1999 | 1.38/100 PY | Patient inclusion criteria: no CD4 criteria; Disease definition: considers only the first episode after cohort enrollment |
| Mocroft, 2000, Lancet | 51 centers in Europe, Eurosida cohort | May 1994 to spring 1999c | Non-ART regimens: 2.3/100 PY; ART regimens: 0.5/100 PY | Patient inclusion criteria: CD4<500 cells/mm3; Disease definition: considers only the first episode after cohort enrollment |
| Baril, 2000, AIDS | Paris, France, Pitié-Salpêtrière Hospital | January 1995 to December 1997 | January 1996 to June 1996: 13.4/100 PY; July 1996 to December 1997: 2.6/100 PY | Patient inclusion criteria: no CD4 criteria; Disease definition: considers the first episode after cohort enrollment |
| Ledergerber, 1999, Journal of the American Medical Association | 7 centers in Switzerland, Swiss HIV Cohort Study | September 1995 to March 1999 | Before ART use: 1.79/100 PY; after ART use: 0.76/100 PY | Patient inclusion criteria: no CD4 criteria, patients who started ART between September 1995 and December 1997. Disease definition: considers only the first episode after cohort enrollmentd |
| Wohl, 2003, Aids Patient Care and STDs | 10 US cities, ASD cohort | 1996c to 2000c | US born: 1.8/100 PY; Mexican born: 1.1/100 PY; Central American born: 0.4/100 PY | Patient inclusion criteria: no CD4 criteria; included US born Latinos, Mexican born Latinos and Central American Latinos. Disease definition: not clear stated, apparently included all OI presented in the study period |
| Low/middle-income settings | ||||
| Badri, 2005, The Southern African Journal of HIV Medicine | Cape Town, South Africa, Cape Town AIDS Cohort (CTAC) | 1992c to December 2000 | 0.40/100 PY | Patient inclusion criteria: no CD4 criteria; Disease definition: disseminated atypical mycobacteria; likely considers all episodes after cohort enrollment |
| Bonard, 2004, AIDS | Ivory Coast, Cotrame ANRS 1203 | 1992c to October 2002 | 1.85/100 PY | Patient inclusion criteria: no CD4 criteria; Disease definition: considers only the first episode after cohort enrollmentd |
| Gadelha, 2002, The Brazilian Journal of Infectious Diseases | Rio de Janeiro, Brazil, IPEC cohort | September 1997 to December 1999 | 0 | Patient inclusion criteria: CD4<100 cells/mm3; Excluded patients under MAC treatment or prophylaxis. Disease definition: considers only the first episode presented in the study period |
ART: highly active antiretroviral therapy; MAC: Mycbacterium avium complex; MSM: men who have sex with men; PCP: Pneumocystis carinii pneumonia.
In HIS, a surveillance study from San Francisco (United States), with no CD4+ cell count restriction of the study population, reported an incidence rate of MAC decreasing from 8.52/100 PY in pre-ART period (1993–1995) to 0.32/100 PY in post-ART period (2001–2008).17 One study from Spain, that included only patients with CD4+ counts less than 500 cells/mm3, reported that the incidence rate of MAC decreased from 2.9/100 PY in pre-ART period (1992) to 0.6/100 PY in post-ART period (1997).11 In addition, data from the Swiss Cohort for patients with CD4+ counts less than 50 cells/mm3, showed an incidence rate of MAC decreasing from 8.8/100 PY in pre-ART period (1990–1996) to 1.4/100 PY in post-ART period (1997–1999).23 Other studies conducted in HIS can be found in Table 4, including results from England, France, Spain, Switzerland, and Germany.
In LMIS, two studies from Africa and one from Brazil report incidence rates of MAC. The South African study, with no CD4+ cell count restriction of the study population reported an incidence rate of 0.4/100 PY for the period of 1992–2000.22 Another study, from Ivory Coast including all HIV-infected found an incidence rate of 1.85/100 PY for the period of 1992–2002.24 The study from Brazil, conducted from 1997 to 1999 among patients with CD4+ cell counts less than 100 cells/mm3 reported no cases of disseminated MAC.25
DiscussionThrough a systematic review of the literature, we have shown that the incidence of opportunistic illnesses decreased over the 30 years of the HIV epidemic, markedly after ART availability. The significant reduction in the incidence rates was demonstrated for opportunistic illnesses overall and also for specific opportunistic infections, namely, PCP, NXT and MAC. In addition, the decreasing trends were shown for both HIS and LMIS where ART was made available. This result is extremely positive as it shows that opportunistic illnesses can be controlled while also pointing to the persistent challenge of a timely diagnosis of HIV infection. Indeed, in order to control opportunistic illnesses HIV infection status must be identified and earlier linkage to care needs to be facilitated. That is, a higher uptake of HIV testing with direct linkage to care of those found to be HIV-infected is urgently needed.
We found that the magnitude of the incidence rates and of the reduction of these rates as a function of ART varied between studies. Indeed, it is well known that there are geographical differences in the incidence of opportunistic illnesses.26 Other reasons for the differences in the baseline rates might include different study populations, including different sociodemographic subgroups evaluated in a specific study, for example, the MACS cohort that focuses on men who have sex men,27 as well as different inclusion criteria. Some studies included all HIV-infected individuals while others restricted the study population to individuals with specific CD4+ cell counts, for example, including only those with CD4+ cell count less than 100 cells/mm3.15 Moreover, different study definitions with respect to the diseases chosen to be included in any given study might have further contributed to the disparate results.
Out of the 37 studies included in the present review, almost 70% were from HIS. Of the 12 studies from LMIS, four studies were from Brazil.13–15,25 These studies reported incidence rates for opportunistic illnesses for the entire study period included in the respective study and not annual rates that could allow us to evaluate the temporal trends in incidence. Also, only one study from Brazil reported separately on the incidence rate of MAC. However, this study reported no cases of the disease, a finding that could have been due to the small sample size and/or short follow-up. For other important diseases that define the AIDS epidemic, namely, PCP, and NXT, no studies from Brazil were found. Furthermore, all are single center cohort studies, two from São Paulo (Hospital das Clínicas, Faculdade de Medicina da Universidade de São Paulo) and two from Rio de Janeiro (Instituto de Pesquisa Clínica Evandro Chagas, Fundação Oswaldo Cruz). We believe that the description of the trends in incidence rates of opportunistic illnesses is of paramount value to health care providers to guide clinical decision-making and policy makers to define priorities for care and prevention of opportunistic infections.
Strengths and limitations to the present study are worth mentioning. Through a systematic review conducted in four databases we found the epidemiological studies that reported on the incidence rate of opportunistic illnesses. We restricted the review to those studies reporting on rates (and not overall numbers or frequencies) since this epidemiological parameter is adjusted for population size and time under risk thus allowing for comparisons between studies. Though not a limitation of our study design and approach, the scarcity of studies from LMIS implies that we cannot adequately describe the patterns of incidence in these settings. In addition, the few studies found should also not be understood as representative of entire countries as they report from one center only. Finally, the different study methodologies such as inclusion criteria and diseases included, for example, limited the comparisons.
In conclusion, the incidence rate of opportunistic illnesses has decreased over time mainly due to the availability of highly effective, safe and well tolerated ART. However, a public health challenge remains for future years. Public health policies focusing on earlier HIV diagnosis and linkage to care, adherence and retention programs, and surveillance of HIV multidrug resistance in populations should be developed and implemented with the goal of improving the quality of life and reducing morbidity and mortality among HIV-infected individuals. To better understand the nuances of the epidemiology of opportunistic illnesses in LMIS, multicenter cohort studies should be encouraged. Finally, it is clear that studies from Brazil are urgently needed to assess the current burden of opportunistic illnesses in order to support the planning of HIV/AIDS health care services organization.
Sources of fundingBG acknowledges funding from the National Council of Technological and Scientific Development (CNPq) and the Research Funding Agency of the State of Rio de Janeiro (FAPERJ). PML acknowledges funding from the National Council of Technological and Scientific Development (CNPq).
Conflicts of interestThe authors declare no conflicts of interest.