Oral pre-exposure prophylaxis (PrEP) with emtricitabine/tenofovir (FTC/TDF) is highly effective in preventing HIV infection. This study aimed to identify factors associated with PrEP early loss to follow-up (ELFU) among gay, bisexual and other men who have sex with men (MSM), travestis and transgender women (TGW).
MethodologyThis was a prospective cohort study evaluating TGW and MSM who initiated PrEP at the Evandro Chagas National Institute of Infectious Diseases (INI-Fiocruz) from 2014 to 2020. ELFU was defined as not returning for a PrEP visit within 180 days after first dispensation. Exposure variables included age, gender, race, education, transactional sex, condomless anal intercourse [CAI] (both in the past six months), binge drinking and substance use (both in past three months) and syphilis diagnosis at baseline. Multilevel logistic regression models with random intercepts and fixed slopes were used to identify factors associated with ELFU accounting for clustering of participants according to their PrEP initiation study/context (PrEP Brasil, PrEParadas, ImPrEP and PrEP SUS).
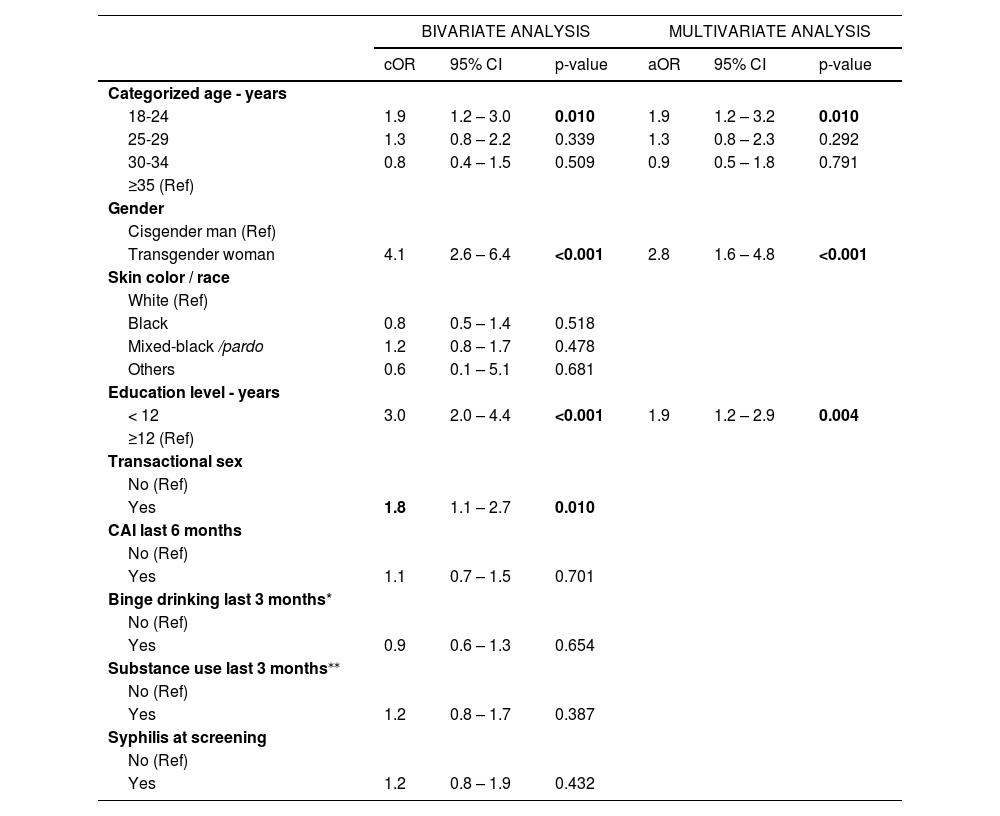
ResultsAmong 1,463 participants, the median age was 29 years (interquartile range 24-36), 83% self-identified as MSM, 17% as TGW, 24% were black, 37% mixed-black/pardo and 30% had < 12 years of education. Fifteen percent reported transactional sex, 59% reported CAI, 67% binge drinking, 33% substance use, and 15% had a syphilis diagnosis. Overall, 137 participants (9.7%) had ELFU. Younger age (18-24 years) (adjusted odds ratio [aOR] 1.9, 95%CI:1.2-3.2), TGW (aOR 2.8, 95%CI:1.6-4.8) and education < 12 years (aOR 1.9, 95%CI:1.2-2.9) were associated with greater odds of ELFU.
ConclusionTGW, young individuals and those with lower education were at higher risk of PrEP ELFU. Our results suggest that the development of specific strategies targeting these populations should be a priority, through policies that aim to reduce the incidence of HIV infection.
In 2021, there were 37.6 million people worldwide living with HIV.1 Transgender women (TGW) and gay, bisexual and other men who have sex with men (MSM) are disproportionally affected by HIV infection, and their risks of acquiring HIV are 34 and 25 times higher than the general population, respectively.1 In Latin America, the estimated prevalence of HIV infection between 2015 and 2019 was 12.6% in MSM and 22.2% in TGW.2 In Brazil, respondent-driven sampling studies estimated HIV prevalence among MSM at 18.4% (multicity study conducted in 2016)3 and 37% among TGW in Rio de Janeiro in 2016.4 Despite available strategies such as treatment as prevention (TasP) implemented in Brazil in 2013 and recommended by the World Health Organization (WHO) since 2014, and pre-exposure prophylaxis (PrEP), recommended by WHO since 2015, the number of new HIV infections in Latin America and the Caribbean rose by 20%, between 2010 and 2020, from 100,000 to 120,000.5
Daily oral PrEP with emtricitabine 200 mg and tenofovir disoproxil fumarate 300 mg (FTC/TDF) significantly reduces the risk of acquiring HIV6,7 and was incorporated in 2017 by the Brazilian Public Health System (Brazilian national PrEP program, known as PrEP SUS program) for individuals at higher risk of acquiring HIV.8 In 2018, a modelling study estimated that PrEP demand across 11 cities in Brazil in the first year of implementation was around 66,000 MSM aged 15 to 64 years.9 However, almost five years after implementation, only 47,821 individuals had initiated PrEP through PrEP SUS in Brazil, of whom just 27,236 were using it by the end of 2021,10 indicating that the number of PrEP users lagged far behind what is needed to reduce new HIV infections. A similar scenario can be described globally. In 2016, the UNAIDS set an ambitious target of three million people on PrEP by 2020, but only 28% of this number was reached due to gaps in PrEP availability, particularly in low and middle income countries.11 In addition to PrEP scale up, attention should be given to PrEP continuation or persistence. Several PrEP implementation programs and dispensing clinics worldwide have reported high rates of PrEP discontinuation that could jeopardize its effectiveness.10,12-15 In the United States (US) and Canada, observational studies reported PrEP discontinuation rates after six months of initiation of 43% and 56%, respectively.16,17 TGW, younger age, black race, substance use (injecting drugs and cannabis), no health insurance, being homeless, sexually transmitted infections (STI) diagnoses, and low number of sexual partners have been associated with PrEP discontinuation in the US.18–20
This study aimed to evaluate PrEP ELFU among TGW and MSM and identify associated factors in the largest HIV prevention service in Rio de Janeiro, Brazil.
MethodsStudy designThis was a cohort study of HIV-negative TGW and MSM aged 18 years or older who initiated PrEP at the INI-Fiocruz in Rio de Janeiro, Brazil from April 2014 to December 2020. Participants included could have initiated PrEP at implementation/demonstration projects (i.e., PrEP Brasil, PrEParadas and ImPrEP) or through the Brazilian PrEP program (PrEP SUS). This study was approved by the INI-Fiocruz institutional review board (CAAE: 52405821.8.0000.5262).
HIV prevention serviceSince 1986, INI-Fiocruz, part of the Brazilian Public Health System, has been a national referral center for research, assistance and academic training in infectious diseases including HIV/AIDS. The HIV prevention and care service is one of the largest tertiary services for people living with HIV in the state of Rio de Janeiro, offering biomedical HIV prophylaxis (pre- and post-exposure prophylaxis) as well as HIV testing and counseling to individuals aged 18 years or older of all genders and sexual orientations at no cost.
Study populationTGW and MSM that initiated PrEP at implementation/demonstration programs (PrEP Brasil, PrEParadas and ImPrEP) or through PrEP SUS were eligible for this study. PrEP eligibility criteria for the implementation/demonstration programs and PrEP SUS were similar and included: age ≥ 18 years, self-identified as cisgender man or TGW, male sex partners, absence of severe kidney impairment (creatinine clearance by using the Cockcroft-Gault formula ≥ 60 milliliters/minute) and reported one or more HIV risk factors in the previous six to 12 months: condomless anal intercourse (CAI), STI diagnosis, sex with partners known to be living with HIV, history of transactional sex (exchanging sex for money, gifts, shelter and drugs) and previous use of post-exposure prophylaxis (PEP). Individuals who reported previous PrEP use were excluded, as well as those with less than 180 days of follow-up since first PrEP dispensation until database closure (December 31, 2020).
Participants included were grouped according to the study they had initiated PrEP in (PrEP Brasil, PrEParadas or ImPrEP) or through the PrEP SUS. Briefly, PrEP Brasil (clinicaltrials.gov register: NCT01989611) was a 48-week PrEP demonstration project conducted between 2014 and 2016 that included 450 MSM and TGW in Rio de Janeiro and São Paulo. Before enrollment all participants underwent a screening visit, and at the enrollment visit participants received their first PrEP dispensation and were followed at weeks 4, 12, 24, 36, and 48 for PrEP provision (tenofovir/emtricitabine) and clinical and laboratory evaluation including HIV testing.21PrEParadas (clinicaltrials.gov register: NCT03220152) was a single site (INI-Fiocruz) study within the PrEP Brasil (same schedule of visits) carried out between 2017 and 2018, which included 130 TGW in order to increase representation of this population in the PrEP Brasil study sample.22ImPrEP (clinicaltrials.gov register: NCT03642314) was a 3-year demonstration project that evaluated same-day PrEP among a total of 4,954 MSM and TGW in Latin America (Brazil: 3,205, Peru: 1,010 and Mexico: 739). PrEP was dispensed at the first visit (there was no screening visit) and participants were followed at weeks 4, 12 and every 12 weeks thereafter (quarterly).23PrEP SUS (Brazilian national PrEP program) was initiated at INI in 2017, users were provided same-day PrEP and followed at weeks 4, and every 12 weeks thereafter.24
PrEP early loss to follow up (ELFU)PrEP ELFU was a dichotomous variable defined as not returning for any PrEP visit within 180 days after the first PrEP dispensation.
Exposure variablesAge at PrEP initiation was categorized in four strata: 18-24, 25-29, 30-34, and ≥35 years; skin color/race was categorized into white, black, mixed-black/pardo and other (Asian and Indigenous); gender was dichotomized into cisgender MSM and TGW; education level was dichotomized into < 12 and ≥ 12 years of education (12 years is equivalent to completing high school education in Brazil).
Behavioral variables included: transactional sex in the previous six months (yes/no); CAI in the previous six months (yes/no); binge drinking (yes/no) defined as drinking five or more doses of alcohol in a couple of hours in the previous three months; and substance use (yes/no), defined as using any of the following in the previous three months: powder cocaine, crack cocaine, cocaine paste, marijuana, amphetamines, club drugs (ketamine, ecstasy, LSD) and GHB.
Definition of syphilis diagnosis at PrEP initiation (yes/no) varied across PrEP demonstration studies and PrEP SUS. In PrEP Brasil and PrEParadas a rapid plasma reagin (RPR) or a Venereal Disease Research Laboratory (VDRL) test was performed at screening; positive results were confirmed by a microhemagglutination assay for Treponema pallidum (MHA-TP). Active/recent syphilis was defined as titers ≥ 1/8 and a positive MHA-TP (WAMA Diagnostic, SP, Brazil).21 In ImPrEP and PrEP SUS syphilis diagnosis at PrEP initiation was defined according to the reverse algorithm that used a treponemal test as the first test TPHA (Hemagglutination for Syphilis), followed by a non-treponemal test VDRL or RPR in the positive results.23
Statistical analysisWe compared PrEP ELFU groups (yes/no) using chi-square test for categorical variables and Kruskal-Wallis Ranksum test for continuous variables. Multilevel logistic regression models with random intercepts and fixed slopes were used to identify factors associated with PrEP ELFU while accounting for the clustering of participants in each group (PrEP Brasil, PrEParadas, ImPrEP and PrEP SUS). Bivariate regression models including the outcome variable and each exposure variables were fit. Exposure variables with p-value <0.20 in the bivariate regression models were included in an initial multivariate regression model. Subsequently, variables with p-value > 0.05 in the multivariate models were removed one by one (backward selection), until reaching the final model. All analyses were performed with R software Version 4.0.3.
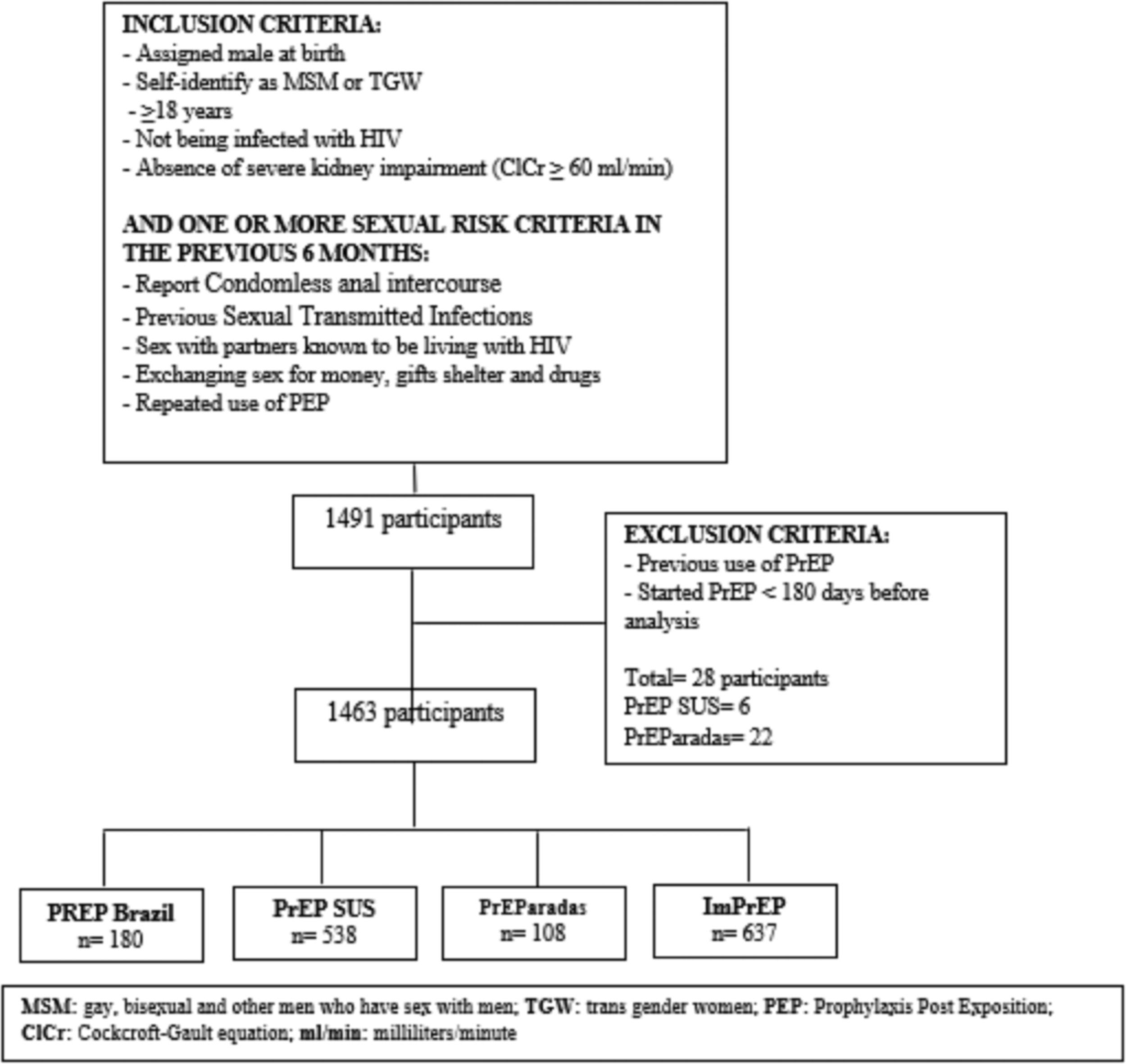
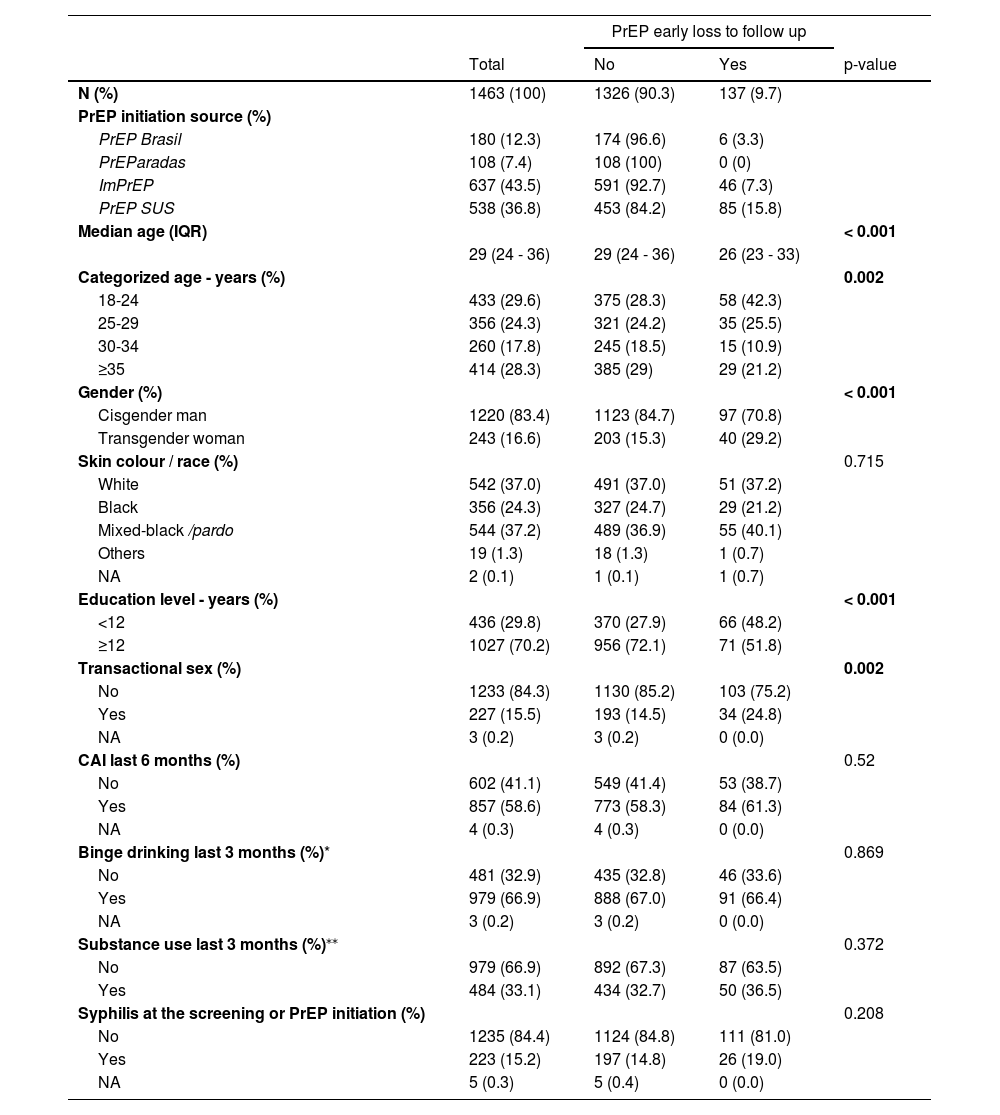
ResultsA total of 1,463 participants were included in the analysis, 180 (12.3%) from PrEP Brasil, 108 (7.4%) from PrEParadas, 637 (43.5%) from ImPrEP and 538 (36.8%) from PrEP SUS. (Fig. 1). The median age was 29 years [interquartile range (IQR) 24-36], 16.6% were TGW, 24.3% self-identified as black and 37.2% as mixed-black/pardo, 70.2% completed ≥ 12 years of education. Transactional sex was reported by 15.5% of the participants, 58.6% reported CAI, 66.9% binge drinking, 33.1% substance use, and 15.2% had a syphilis diagnosis at PrEP initiation (Table 1).
Characteristics of participants who initiated PrEP at a large HIV prevention service from 2014 to 2020 in Rio de Janeiro, Brazil.
| PrEP early loss to follow up | ||||
|---|---|---|---|---|
| Total | No | Yes | p-value | |
| N (%) | 1463 (100) | 1326 (90.3) | 137 (9.7) | |
| PrEP initiation source (%) | ||||
| PrEP Brasil | 180 (12.3) | 174 (96.6) | 6 (3.3) | |
| PrEParadas | 108 (7.4) | 108 (100) | 0 (0) | |
| ImPrEP | 637 (43.5) | 591 (92.7) | 46 (7.3) | |
| PrEP SUS | 538 (36.8) | 453 (84.2) | 85 (15.8) | |
| Median age (IQR) | < 0.001 | |||
| 29 (24 - 36) | 29 (24 - 36) | 26 (23 - 33) | ||
| Categorized age - years (%) | 0.002 | |||
| 18-24 | 433 (29.6) | 375 (28.3) | 58 (42.3) | |
| 25-29 | 356 (24.3) | 321 (24.2) | 35 (25.5) | |
| 30-34 | 260 (17.8) | 245 (18.5) | 15 (10.9) | |
| ≥35 | 414 (28.3) | 385 (29) | 29 (21.2) | |
| Gender (%) | < 0.001 | |||
| Cisgender man | 1220 (83.4) | 1123 (84.7) | 97 (70.8) | |
| Transgender woman | 243 (16.6) | 203 (15.3) | 40 (29.2) | |
| Skin colour / race (%) | 0.715 | |||
| White | 542 (37.0) | 491 (37.0) | 51 (37.2) | |
| Black | 356 (24.3) | 327 (24.7) | 29 (21.2) | |
| Mixed-black /pardo | 544 (37.2) | 489 (36.9) | 55 (40.1) | |
| Others | 19 (1.3) | 18 (1.3) | 1 (0.7) | |
| NA | 2 (0.1) | 1 (0.1) | 1 (0.7) | |
| Education level - years (%) | < 0.001 | |||
| <12 | 436 (29.8) | 370 (27.9) | 66 (48.2) | |
| ≥12 | 1027 (70.2) | 956 (72.1) | 71 (51.8) | |
| Transactional sex (%) | 0.002 | |||
| No | 1233 (84.3) | 1130 (85.2) | 103 (75.2) | |
| Yes | 227 (15.5) | 193 (14.5) | 34 (24.8) | |
| NA | 3 (0.2) | 3 (0.2) | 0 (0.0) | |
| CAI last 6 months (%) | 0.52 | |||
| No | 602 (41.1) | 549 (41.4) | 53 (38.7) | |
| Yes | 857 (58.6) | 773 (58.3) | 84 (61.3) | |
| NA | 4 (0.3) | 4 (0.3) | 0 (0.0) | |
| Binge drinking last 3 months (%)* | 0.869 | |||
| No | 481 (32.9) | 435 (32.8) | 46 (33.6) | |
| Yes | 979 (66.9) | 888 (67.0) | 91 (66.4) | |
| NA | 3 (0.2) | 3 (0.2) | 0 (0.0) | |
| Substance use last 3 months (%)⁎⁎ | 0.372 | |||
| No | 979 (66.9) | 892 (67.3) | 87 (63.5) | |
| Yes | 484 (33.1) | 434 (32.7) | 50 (36.5) | |
| Syphilis at the screening or PrEP initiation (%) | 0.208 | |||
| No | 1235 (84.4) | 1124 (84.8) | 111 (81.0) | |
| Yes | 223 (15.2) | 197 (14.8) | 26 (19.0) | |
| NA | 5 (0.3) | 5 (0.4) | 0 (0.0) | |
IQR interquartile range; NA Not available (missing values); CAI Condomless anal intercourse.
Overall, 137 participants (9.7%) met the ELFU definition (95%CI 0.08-0.11) (Table 2). Participants with ELFU were younger (median age 26 [IQR 23-33] vs. 29 years [IQR 24-36], p < 0.001), more likely to be TGW (16.4% vs. 8%, p < 0.001) and were less educated (15.1% vs. 6.9% had < 12 years of education, p < 0.001) compared to non-ELFU. A higher proportion of transactional sex was also observed in the ELFU group compared to the non-ELFU group (14.9 vs. 8.4%, p = 0.002). The proportion of participants reporting CAI, binge drinking and stimulants use, as well as a syphilis diagnosis was similar between the two groups.
Factors associated with PrEP early loss to follow up at the INI's HIV prevention service from 2014 to 2020.
| BIVARIATE ANALYSIS | MULTIVARIATE ANALYSIS | |||||
|---|---|---|---|---|---|---|
| cOR | 95% CI | p-value | aOR | 95% CI | p-value | |
| Categorized age - years | ||||||
| 18-24 | 1.9 | 1.2 – 3.0 | 0.010 | 1.9 | 1.2 – 3.2 | 0.010 |
| 25-29 | 1.3 | 0.8 – 2.2 | 0.339 | 1.3 | 0.8 – 2.3 | 0.292 |
| 30-34 | 0.8 | 0.4 – 1.5 | 0.509 | 0.9 | 0.5 – 1.8 | 0.791 |
| ≥35 (Ref) | ||||||
| Gender | ||||||
| Cisgender man (Ref) | ||||||
| Transgender woman | 4.1 | 2.6 – 6.4 | <0.001 | 2.8 | 1.6 – 4.8 | <0.001 |
| Skin color / race | ||||||
| White (Ref) | ||||||
| Black | 0.8 | 0.5 – 1.4 | 0.518 | |||
| Mixed-black /pardo | 1.2 | 0.8 – 1.7 | 0.478 | |||
| Others | 0.6 | 0.1 – 5.1 | 0.681 | |||
| Education level - years | ||||||
| < 12 | 3.0 | 2.0 – 4.4 | <0.001 | 1.9 | 1.2 – 2.9 | 0.004 |
| ≥12 (Ref) | ||||||
| Transactional sex | ||||||
| No (Ref) | ||||||
| Yes | 1.8 | 1.1 – 2.7 | 0.010 | |||
| CAI last 6 months | ||||||
| No (Ref) | ||||||
| Yes | 1.1 | 0.7 – 1.5 | 0.701 | |||
| Binge drinking last 3 months* | ||||||
| No (Ref) | ||||||
| Yes | 0.9 | 0.6 – 1.3 | 0.654 | |||
| Substance use last 3 months⁎⁎ | ||||||
| No (Ref) | ||||||
| Yes | 1.2 | 0.8 – 1.7 | 0.387 | |||
| Syphilis at screening | ||||||
| No (Ref) | ||||||
| Yes | 1.2 | 0.8 – 1.9 | 0.432 | |||
INI's: Instituto Nacional de Infectologia Evandro Chagas. CAI Condomless anal intercourse.
ELFU varied across the different PrEP implementation studies and PrEP SUS. The highest ELFU was in the PrEP SUS group (15.8%, 95% CI 0.1-0.2), followed by ImPrEP (7.3%, 95% CI 0.05-0.1) and PrEP Brasil (3.3%, 95% CI 0.01-0.07) with no ELFU participants from the PrEParadas group (0.0%). Among TGW, overall ELFU was 29.2% (95%CI 0.1-0.2) and varied from 36.5% (95%CI 0.3-0.5) in PrEP SUS to 19.6% (95%CI 0.1-0.3) in ImPrEP. There were no TGW included in PrEP Brasil and no TGW in PrEParadas met the ELFU definition.
In a final multivariate model, younger age (18-24 years) (adjusted odds ratio [aOR] 1.9, 95%CI 1.2-3.2), TGW (aOR 2.8, 95%CI 1.6-4.8), and having lower education (aOR 1.9, 95%CI 1.2-2.9) were associated with greater odds of ELFU.
DiscussionWe found an overall 9.7% ELFU among TGW and MSM enrolled to receive daily oral FTC/TDF PrEP in demonstration/implementation studies and through routine prevention services at INI-Fiocruz between 2014-2020. Moreover, we found higher ELFU in routine PrEP care (PrEP SUS) compared to demonstration projects, and among same-day PrEP initiators (those within PrEP SUS and ImPrEP) relative to demonstration studies that conducted a screening visit before PrEP dispensation (PrEP Brasil and PrEParadas). In this study, younger age, being TGW and having lower educational level were independently associated with ELFU.
Estimates of PrEP loss to follow-up or discontinuation vary among different studies and contexts. A real-life study conducted in the US between 2012 and 2017 estimated 16% and 46% of early or late PrEP discontinuation (< 90 vs. ≥ 90 days after PrEP initiation), respectively.13 Also in the US, a national survey carried out in 2017 identified 49% of PrEP discontinuation among individuals who had ever used PrEP.12 In Brazil, analyses from the national PrEP SUS program (2018-2021) found a discontinuation rate of 43% considering not returning to the scheduled appointment within 1, 2, 3 or 4 months.10 Conversely, data from implementation projects have described much lower rates of loss to follow-up or discontinuation among PrEP users than real-life estimates. In Brazil, the PrEP Brasil implementation project reported a non-retention rate of 17% at 48 weeks after PrEP initiation.21 In Australia, an implementation study found a discontinuation rate of 30% at nine months after PrEP initiation;14 and a Spanish implementation study reported an abandonment rate of 11.9% at 12 months (defined as failure to attend any visit at weeks 4, 12, 24, 36, 48, 52). This low estimate was attributed to the fact that most of the cohort participants had a university degree and strict control of the study visit schedule.15,25 Altogether, the evidence suggests that the high PrEP persistence and retention observed in early PrEP implementation studies were not maintained in routine clinical settings.13 Our results reinforce these findings, with higher ELFU estimates for PrEP SUS users relative to PrEP users of implementation studies. Delay in PrEP initiation > 30 days was associated with shorter PrEP persistence, simplified processes for starting PrEP led to better persistence.26 Although the same day PrEP strategy has been shown to be safe and convenient, a study conducted at the Denver Metro Health Clinic found that 43% of users missed follow-up appointments within 180 days,27 suggesting that same day PrEP initiation may also explain the disparities in ELFU estimates observed in PrEP SUS and ImPrEP compared to PrEP Brasil.
Our study found a three-fold greater odds of ELFU for TGW than for MSM. It is worth mentioning that our service has gender-neutral spaces aiming to engage and facilitate retention of TGW in care.28 A similar finding was reported in a study that evaluated PrEP discontinuation in San Francisco primary care clinics (2012-2017), where the risk of PrEP discontinuation in three months was twice higher among TGW compared to MSM.13 Our results are worrisome as TGW bear a disproportionately high burden of HIV. In Rio de Janeiro, a respondent-driven sampling study (Transcender) estimated the prevalence of HIV among TGW as 32.1%.29 Despite being one of the populations that would most benefit from PrEP, in Brazil only 3.3% of all PrEP users within the national PrEP SUS program are TGW,10 a small fraction that can be explained by several factors. First, TGW have low access to health care and transphobia and discrimination are listed by them as the main barriers to access health services. Second, PrEP knowledge and awareness levels are low among TGW; in Rio de Janeiro, Transcender found that only 38% of the TGW had ever heard about PrEP, and lower education and not having accessed health care in the previous six months were associated with low PrEP awareness.30 Stigma, discrimination, reluctance to know their HIV status, fear of risk compensation with subsequent abandonment of condom use, the fact that its use would not reduce the concern of becoming infected and the lack of trans-specific care were also identified as PrEP barriers among TGW in Brazil.31-33 Importantly, among the 108 TGW enrolled in PrEParadas, none met the ELFU definition. This finding can be explained by the study's strict schedule and efforts to retain participants. Moreover, the PrEParadas study offered feminization hormone therapy, mental health and endocrinological care at no cost for all participants; highlighting that gender affirming health care can improve PrEP retention.22
Younger participants aged 18-24 years old were two-fold more likely for PrEP ELFU than those aged ≥ 35 years. Higher risk of ELFU among young individuals has been reported in other settings. In Brazil, national data from PrEP SUS program showed that younger individuals (18-24 years old) had twice higher odds of discontinuing PrEP in the first month after PrEP initiation.34 Similarly, in a Thai study carried out between 2016 and 2019, individuals aged < 20 had 1.7 higher odds of PrEP discontinuation in the first month.35 In the US (multicity PrEP demonstration study conducted in 2012-2014) younger age was associated with early loss of retention and intermittent retention.36 The WHO estimated that in 2019, two out of seven new HIV infections globally were among young people (age 15-24); and the HIV prevalence among young transgender people and young MSM was estimated at 11% and 6%, respectively.37 In Latin America, a high prevalence of HIV infection (>10%) among young MSM was found in different countries.3,38-41 Young MSM are more vulnerable to HIV compared to their older peers due to riskier sex behavior, higher frequency of transactional sex and use of illicit drugs.42 On the other hand, young MSM have a lower HIV risk perception, less PrEP awareness and a lower willingness to use PrEP when compared to older MSM.43 Studies addressing barriers to PrEP use and continuation specifically among young MSM and TGW are currently ongoing in Rio de Janeiro, aiming to evaluate strategies tailored specifically for young MSM and young TGW to support PrEP use in these groups.44,45
Finally, our analyses found that participants with lower education levels (up to secondary education) had almost twice higher odds of ELFU than those with ≥ 12 years of education. In a Thai study (2016-2019), a similar negative association between low education and PrEP loss to follow up was reported.35 Moreover, low education has been described as a risk factor for HIV acquisition. In China, a systematic review that estimated HIV burden among MSM (2001-2018) found that the HIV prevalence decreased with increasing years of education (16.8% and 5.7% among the illiterate and those with a college education, respectively).46 In Rio de Janeiro (2018-2020), a cross sectional study evaluating incidence of HIV using a recent infection testing algorithm (RITA), found that the estimated annualized incidence rate of HIV infection was 1.5 times higher among TGW and MSM with less than 12 years of education than in those with higher education (9.5% vs. 6.5%).47 Furthermore, a Brazilian national web-based survey revealed that lower education was associated with higher chances of self-report being HIV positive.48 In addition, in Rio de Janeiro, TGW with more than eight years of education had an adjusted odds 1.5 times greater of knowing about PrEP.30
Our study had several limitations. First, there could be residual confounding from unmeasured variables (i.e., individuals income, employment status).49 Second, INI-Fiocruz is a large referral center for HIV care and prevention, and the estimated ELFU PrEP observed in our study may not be generalizable to other clinical settings. Third, our study was a post-hoc analysis of previous studies carried out at INI-Fiocruz and included MSM and TGW initiating PrEP in different contexts (implementation studies as well as routine PrEP care). To address this potential source of heterogeneity, multilevel regression models were used. Finally different definitions have been used for PrEP loss to follow-up or discontinuation in the literature, with varying observation periods (i.e., 30 days, 90 days, 180 days) and outcome definitions (i.e., missed visits, PrEP refill), underscoring a need for cautious comparison of estimates.35,50 Nonetheless, our study has unique strengths. The study population included a large sample of MSM and TGW initiating PrEP in Brazil from 2014 to 2020, including a large group of 200 TGW, and providing crucial data to build knowledge on PrEP ELFU in Brazil.
Since July 2022, the WHO recognized that long-acting injectable cabotegravir may be offered as an additional prevention choice for people at substantial risk of HIV infection.51 Long-acting cabotegravir has been proven efficacious in reducing the risk of HIV infection in randomized clinical trials.52,53 Moreover, the offer of additional PrEP choices (i.e., long acting injectables) has the potential of increasing uptake and effectiveness of PrEP. In Brazil, an implementation study of long-acting cabotegravir has been recently launched and will include participants in six different cities to evaluate real life effectiveness and implementation strategies (Clinical trials gov number NCT05515770).
ConclusionsOur results revealed populations with higher odds of PrEP ELFU including TGW, young individuals and those with lower education. These same populations share a disproportionate high risk of HIV acquisition. Moreover, we found high ELFU among same-day PrEP users in routine PrEP care. Identification of barriers to PrEP continuation as well as providers-driven tailored strategies for these high-risk groups are needed to improve PrEP retention in care. Further studies aiming to investigate interventions (i.e., technology-based or peer navigation-based) to facilitate PrEP use among TGW and young individuals are ongoing and could lead to new tools to improve PrEP outcomes in these populations.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. PrEP Brasil was sponsored by the Brazilian Ministry of Health, Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ) and Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP). ImPrEP project was made possible thanks to Unitaid's funding and support. Unitaid accelerates access to innovative health products and lays the foundations for their scale-up by countries and partners. Unitaid is a hosted partnership of WHO. Imprep was also supported by the Brazilian Ministry of Health.
We are grateful to the study participants and the study teams of PrEP Brasil, PrEParadas, ImPrEP and the team of PrEP SUS.