Human Papillomavirus (HPV) is the main etiological factor for the development of cervical cancer. HPV 18 is the second most frequent type, accounting for up to 65% of all cases. HPV intratypic variation may influence the potential for progression to invasive cancer. The aim of this study was to evaluate the prevalence of human papillomavirus 18 intratypic variants in cervical cancer samples from women in the state of Maranhão, Brazil.
MethodsThe study included 118 women over 18 years of age with a diagnosis of cervical cancer. Tumor fragments were collected and subjected to DNA extraction and Polymerase Chain Reaction (PCR) for HPV detection using the PGMY09/11 and GP+5/6 primers. Positive samples were submitted to automated sequencing for viral genotyping. To determine the HPV 18 lineages, positive samples were submitted to PCR, using specific primers to amplify the LCR and E6 regions of HPV 18 virus.
ResultsHPV was present in 88 women (73.3%). Of those, 48 (54%) were HPV 16, the most prevalent, followed by 12 (13.6%) HPV 18. Histologically, squamous cell carcinoma was predominant (79.1%). Among the HPV 18 variants identified, 10 (80%) belonged to lineage A, and sublineages A1, A2, A3, and A4. Two (29%) HPV 18 B variant was also detected, with the sublineages B1 and B2. In this study, the C variant was not found. There was no statistically significant association between the HPV 18 lineages found and sociodemographic and lifestyle variables (p > 0.05).
ConclusionsA higher frequency of HPV 16 and 18 were found in women with cervical cancer in the state of Maranhão, Brazil, with a high prevalence of the lineage A among women with HPV 18.
Cervical cancer is the fourth most common type of cancer among women in the world.1 Each year, 527,624 women are diagnosed with cervical cancer and 265,672 die from this disease.1 According to the National Institute of Cancer (INCA), 16,370 new cases of cervical cancer were estimated to occur each year in Brazil in the biennium 2018–2019, with an approximate risk of 15.43 cases per 100,000 women.2 Most cases of cervical cancer occur in Africa, Latin America and Asia, where for every nine cases of cancer, at least one is cervical cancer.3 In developed North American and European countries, the incidence of cervical cancer is less than nine per 100,000 inhabitants.4 In developing countries, less than 50% of women affected by cervical cancer survive for more than five years compared to 66% in developed countries.5 This type of malignant neoplasm presents a variety of histological types, with squamous cell carcinoma accounting for up to 95% of cases, followed by adenocarcinoma and adenosquamous carcinoma.6
Human papillomavirus (HPV) infection is the main etiologic factor for cervical cancer, with more than 200 types already identified and classified according to their oncogenic potential.7 Among subtypes, HPV 18 is the second most frequent, accounting for up to 65% of all cases of invasive cervical cancer worldwide.8 Studies have reported that HPV 18 is closely associated with the development of glandular lesions and adenocarcinoma (ADC) compared to squamous cell carcinoma (SCC).8 This finding indicates that HPV 18 shares phylogenetic traits that favor the development of adenocarcinoma compared to other HPV genotypes.8
HPV intratypic variants show different distribution and specificity in various parts of the globe. These variants are characterized based on the isolation of the same viral type and have a difference of less than 10% in the nucleotide sequence of the L1 region of the virus.9 These differences promote changes in pathogenicity and oncogenic potential between variants of the same viral type.10 Studies suggest that different HPV 18 variants co-evolved with the three major human phylogenetic branches, namely African, Caucasian, and Asian. In view of this, the variants were classified into three distinct lineages, the African (AF), the European (E), and Asian-Amerindian (As/Ai).8,10 Currently, a new nomenclature classifies HPV 18 variants in three lineages: A (including the lineages previously classified as As/Ai and E), B, and C (previously classified as lineage Af).8 In addition to classification in variants (up to 10% divergence), there is the classification into sublineages, when a divergence of 0.5 to 1% occurs between the same variant. For HPV 18, the sublineages are classified into: variant A (A1, A2, A3, A4 and A5), variant B (B1, B2 and B3), and variant C (has no sublineages).11
Genetic variations in the same viral type may influence the potential for infection, viral persistence, development of precursor lesions, and progression to invasive cancer.9,12 Compared to studies conducted with HPV 16, few reports have described the frequency of invasive cervical cancer associated with HPV 18 infection. It is therefore necessary to develop researches to understand their association with pathological and oncogenic aspects of cervical lesions.8,9 The objective of this study was to evaluate the prevalence of HPV 18 intratypic variants in cervical cancer samples in women assisted in the Northeast of Maranhão state, Brazil.
MethodsStudy designThis was a descriptive, cross-sectional study. The research sample consisted of 118 women older than 18 years with diagnosis of cervical cancer, treated at a reference oncology hospital in the state of Maranhão, between January 2016 and June 2017. This study was approved by the Research Ethics Committee of the Federal University of Maranhão - UFMA under number 1.289.419/2015. The women who agreed to participate in the study signed an Informed Consent Form (ICF). Standardized questionnaires were used to collect sociodemographic information of the patients.
Sample collection, DNA extraction, PCR for HPV detectionFresh tumor biopsies were collected and stored in Later RNA at −80 °C. DNA extraction was performed using the QIAmp DNA Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer's instructions. The presence of HPV DNA was detected by the nested polymerase chain reaction (PCR) technique, with a first round of reactions with the PGMY09/11 primers, and a second with the GP+5/6 primers, amplifying the sequence of the L1 region of the virus genome.
Sequencing to identify HPV typesPCR products from positive samples were purified using the DNA and Gel Band Purification kit (GE Healthcare) and sequenced using the Big Dye Terminator v3.1 Cycle Sequencing kit (Applied Biosystems) on ABI Prism3130XL Genetic Analyzer (Applied Biosystems). Chromatograms of the sequences obtained were initially analyzed and edited in the 4Peaks software (Nucleobytes, Amsterdam, Netherlands). The sequences were then submitted to the online BLASTn software (Basic Local Alignment Search Tool, http://blast.ncbi.nlm.nih.gov/) for the identification of HPV types.
Identification of HPV 18 variantsTwo pairs of initiators were used to amplify the entire CSF region and the E6 gene, specific for HPV 18. The reaction was performed in 25 μL of a mix containing 1X PCR Buffer, 2.5 mM MgCl2, 0.2 μM of each DNTP, 100 μmol/L of each primer, 50–100 ng of DNA, and 2.5 U of Platinum Taq DNA Polymerase (Life Technologies). Amplification was performed as follows: 95 °C for 10 min; followed by 40 cycles of 1 min at 95 °C, 1 min at annealing temperature, and 1 min at 72 °C, followed by a final extension at 72 °C for 15 min. Consensus sequences were aligned according to lineage-specific reference sequences suggested by Burk (2013) using the Mega software (version 6, www.megasoftware.net). Then, in the step of PCR reactions, the products generated were subjected to the same purification protocols and direct sequencing for identification of HPV type.
Editing, sequence assembly, and phylogenetic analysisHPV 18 lineages were identified according to high-quality electropherograms of the LCR and E6 sequences and characterized according to lineage-distinctive single-nucleotide variations (SNVs) of HPV 18 CSF and E6 sequences. Sequence alignment was performed using the MEGA software 5.2. Phylogenetic analysis of HPV 18 sequences was done using the Maximum Likelihood method in the PHYML software and the GTR model. Bootstrap values were estimated after 1000 replications to evaluate the support of the internal branches. Phylogenetic reconstruction was performed using 1300 bp sequence data from the LCR and E6 regions, including reference sequences suggested by Burk et al.13
Statistical analysisDescriptive statistical analysis was performed in the Stata software (version 14.0), and data were presented in the form of figures and tables. The chi-square test was used to check the association between HPV and sociodemographic and clinical variables; p-values ≤ 0.05 were considered statistically significant. The Fisher's exact test was used to verify the association between HPV18 and clinical variables; p-values ≤ 0.05 were considered statistically significant.
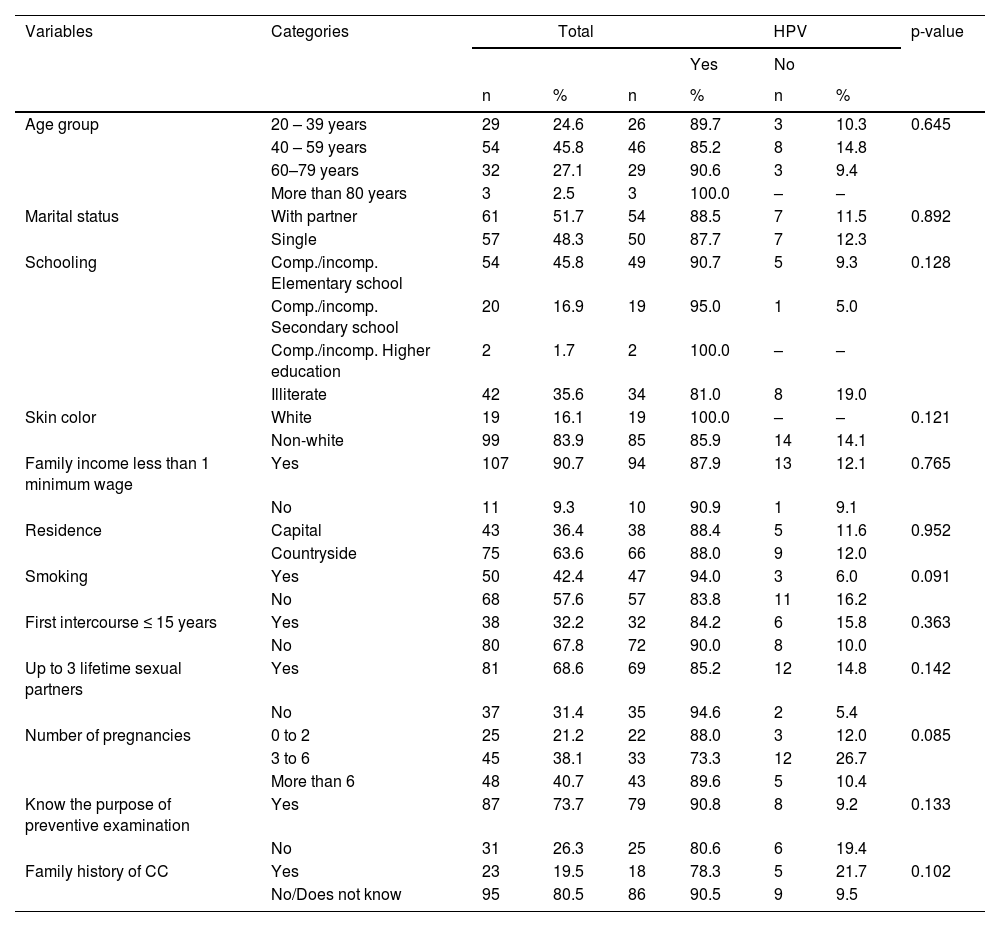
ResultsCharacterization of the populationTable 1 shows the sociodemographic data of 118 patients diagnosed with cervical cancer assisted at reference oncology hospitals in the state of Maranhão. Of these, 88 (73.3%) had HPV infection, 48 (54%) HPV 16, the most prevalent, followed by 12 HPV 18 (13.6%). Viral types HPV35, HPV33, HPV45, HPV52, HPV58, HPV59, HPV53, HPV31 were also found in this cohort. Squamous cell carcinoma (79.1%) was the predominant tumor type. The majority of the study sample consisted of self-reported non-white women (83.9%), aged between 40 and 49 years (45.8%), married or common-law married (51.7%), with elementary school (45.8%), and monthly income less than one minimum wage (90.7%).
Sociodemographic characteristics and risk factors of cervical cancer patients. São Luís, state of Maranhão, Brazil, 2016–2017.
CC, cervical cancer.
Regarding risk factors for HPV infection, 57.6% of the women reported being non-smokers, 67.8% had more than three sexual partners in a lifetime, 40.6% had more than three pregnancies, 73.7% knew the purpose of the Papanicolaou test in CC prevention, and 80.5% had no family history of cervical cancer. There was no statistically significant association between the presence of HPV and sociodemographic and lifestyle variables among cervical cancer women (p > 0.05).
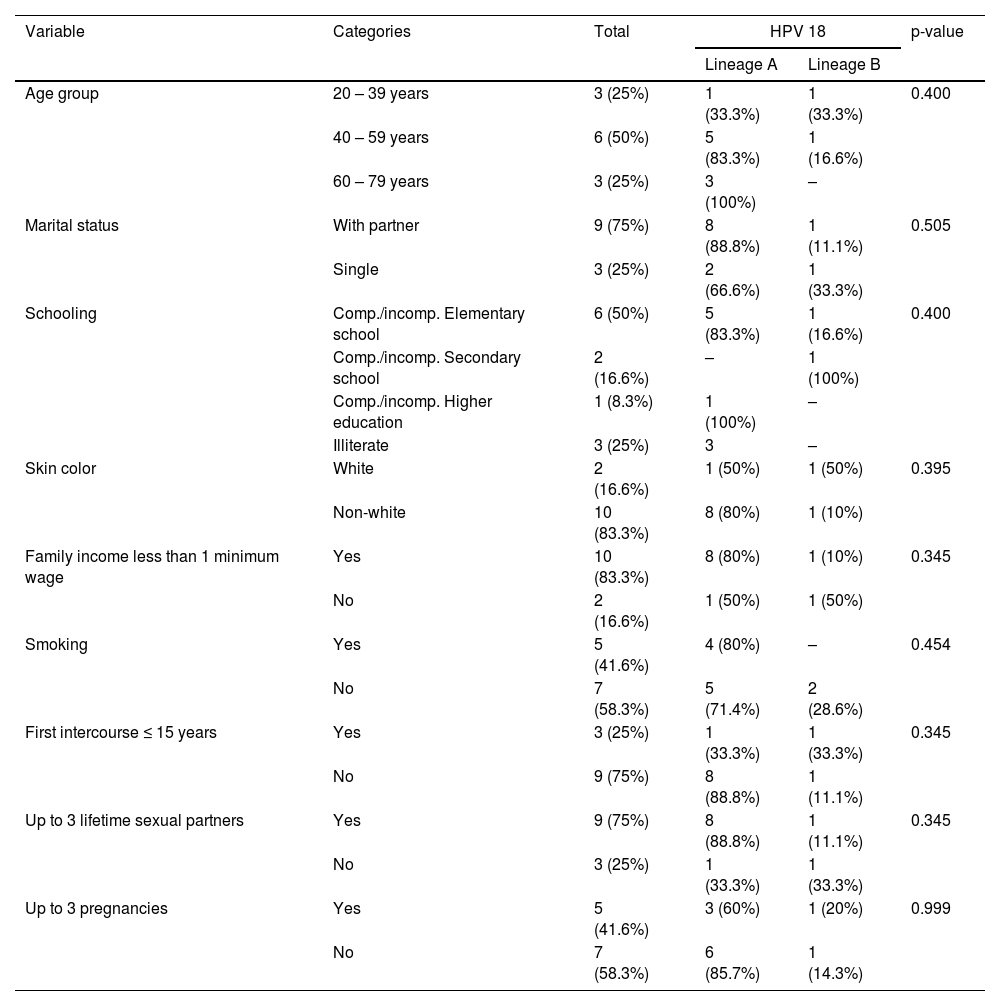
Characterization of HPV 18 positive womenTable 2 reports the sociodemographic data of HPV 18 positive women. The majority of HPV 18 infected women were aged between 40 and 49 years (54.5%), married or common-law married (75%), with schooling up to complete or incomplete elementary education (50%), and had a monthly income less than 1/2 minimum wage (83.3%).
Sociodemographic and clinical characteristics of HPV 18 infected patients with cervical cancer. São Luís, state of Maranhão, Brazil, 2016–2017.
*We were unable to identify the HPV 18 lineage of one patient.
Most of HPV 18 positive women reported being non-white skin color, non-smokers (58.3%), having less than three sexual partners in a lifetime (75%), and having more than three pregnancies (58.3%). There was no statistically significant association between the HPV 18 lineages and sociodemographic and lifestyle variables among cervical cancer women (p > 0.05).
HPV 18 variantsHPV 18 variants were sequenced from PCR products, corresponding to the E6 and LCR regions of the viral genome. Of the 12 women with HPV 18 infection, 10 presented the variant A (83.3%) and two the variant B (16.7%). Sublineages A1, A2, A3 and A4 were identified in the case of lineage A. As for the lineage B, the sublineages B1 and B2 were identified. The lineage C of HPV 18 was not documented in this study.
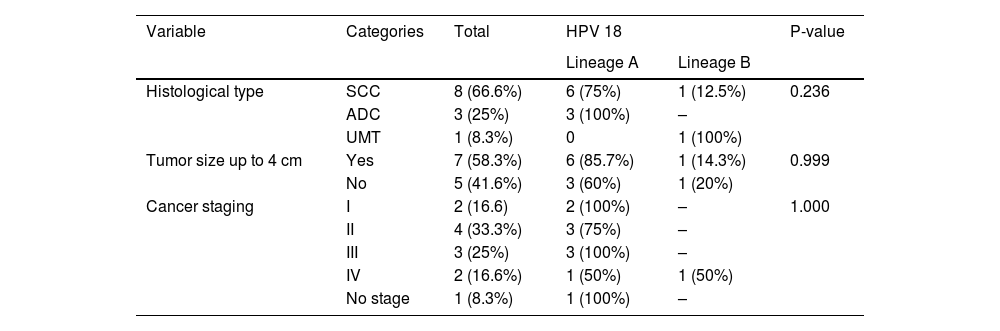
Table 3 shows data regarding histological aspects of tumors of HPV 18 infected women. The most prevalent type was squamous cell carcinoma - SCC (66.7%), followed by adenocarcinoma (25.0%). Among HPV 18 lineage A positive women, 66.7%% presented SCC and 33.3% adenocarcinoma tumors. Among the cases of adenocarcinoma, all (100%) had lineage A. There was no statistically significant association between these variables.
Characteristics of cervical tumors in women who presented HPV 18 infection. São Luís, state of Maranhão, Brazil, 2016–2017.
SCC, squamous cell carcinoma, ADC, adenocarcinoma, UMT, undifferentiated malignant tumor.
*Two patients with no data on HPV 18 lineage had histological type squamous cell carcinoma (SCC).
** One patient with HPV 18 lineage A were not evaluated for cancer staging.
*** One patient with no data on HPV 18 lineage had cancer stage II.
Regarding the size of the tumor, 58.3% of the cases had tumors smaller than or equal to 4 cm, of which 85.7% belonged to lineage A. Cases in which the tumor was larger than 4 cm, 60% belonged to the lineage A. The same was observed in relation to the staging of the disease, where stages II and III were predominant, with 33% and 25%, respectively. HPV 18 lineage A was also predominant among these cases. There was no statistically significant association between HPV 18 lineages and histopathological variables (p > 0.05).
DiscussionThere was a high prevalence of HPV in cervical tumor samples of women resident in the Northeast of Brazil in this study. HPV 16 and 18 were the most common subtypes, similar to other studies carried out in Brazil.14-17
Regarding HPV 18 infected women, our data show high prevalence of lineage A, followed by lineage B, while lineage C was not found. Regarding the sublineages, the A1, A2, A3 and A4 were documented for lineage A. In turn, the sublineages B1 and B2 were found for the lineage B. Moreover, our data show that cervical cancer women infected with HPV 18 had similar sociodemographic data as women infected with other virus types, mainly low-level of education, low-income, and residence in the countryside. We have also showed that there was no statistically significant association between HPV presence and sociodemographic variables in women with cervical cancer.
Regarding the characteristics of cervical tumors, squamous cell carcinoma was predominant in both HPV 18 and other HPV-types infected women, as well as in HPV 18 lineage A. Regarding staging and tumor size, both HPV 18 lineage A and B tumor samples were primarily staging 2 and 3, with tumor size up to 7 cm.
The prevalence of HPV in cervical tumor samples may range from 70 to 100% depending on the technique used for virus detection. Other studies have indicated that the four most prevalent types of HPV in South and Central America are HPV 16, 18, 31, and 45.18,19 The HPV 16 and 18 variants have been extensively studied to analyze their role in the development of cervical intraepithelial neoplasms and progression to invasive cervical cancer, taking into account the geographical and ethnic influence in the distribution of variants worldwide.8,11,14 Specific variations of HPV 18 may present high oncogenic potential and influence the variation of distribution of cervical cancer in different regions.20
Few studies with HPV 18 variants have been carried out until present, preventing comparisons of the present findings with other studies and regions. Some studies reported that the lineage A of HPV 16 and HPV 18 are more prevalent in European populations, while the lineage D of HPV 18 predominates in populations with Amerindian ancestry. The lineages B and C are more prevalent in the African continent.14,21 Most studies have been conducted in women with cervical intraepithelial lesions, also showing a high prevalence of the lineage A. Previous studies reported a high risk of cervical intraepithelial lesions in women with European or Asian HPV 18 variants. It has also been reported that infection by European HPV 18 variants were more likely to become persistent. However, a study by Villa et al. (2000) indicated that infection by non-European variants presented a greater chance of persistence of infection and association with pre-invasive lesions.20,22 Thus, more research is needed to understand which virus lineages may be associated with persistence and progression of cervical lesions.
The population of Maranhão is made up of people of different genetic background, with up to 75% African ancestry, besides Amerindian and indigenous.23 Due to the high contribution of African offspring, it was expected that there would be a greater distribution of HPV 18 B and C lineages in the population of Maranhão. The low prevalence of these lineages may be associated with intense ethnic mixture, with individuals presenting varying proportions of several ancestries, or with the level of carcinogenic potential in the studied population.14,21,24 Other studies in Brazil were carried out to investigate the HPV 16 lineages in Brazilian regions, but this is the first study to identify the HPV 18 lineages in the population of Maranhão.
In a study conducted in Brazil by Vidal et al. (2016), a cohort of 71 women with cervical cancer infected with HPV 18 had a higher prevalence of lineage A followed by lineage B; lineage C was not found either.14 A study carried out by Xu et al. (2018) with 138 women diagnosed with HPV 18 infection sought to identify the variants present in the population of Taizhou, China. The study concluded that all HPV 18 variants found belonged to the A lineage, while B and C lineages were not found in that population.8
In a study conducted in the Netherlands by King et al. (2016) with 835 male and female subjects, HPV 18 was identified in 108 individuals. Of these, 86% belonged to variant A, 14% belonged to variant B. The (non-European) variant C was also identified in 15 samples, most of which were taken from non-Dutch individuals.25 In a study by Chen et al. (2015) with 711 HPV 18 positive samples from 39 countries, there was predominance of variant A in all regions, except in sub-Saharan Africa where variant B prevailed. Variant C occurred only in Africa. In samples belonging to variant A, the sublineage A1 (248 samples) was predominant. Similarly, the sublineage B1 (46 samples) prevailed in the variant B.11
In Brazil, HPV vaccine was incorporated into the National Immunization Program (NIP) in 2014.26 The target population are girls aged 9 to 14 years and boys aged 11 to 14 years.27 In the state of Maranhão, only 70.8% of girls and 47.5% of boys were vaccinated.28
Studies also indicate a variation in the distribution of HPV types associated with histological types. HPV 16 has been associated with SCC, whereas HPV 18 has a higher prevalence in ADC.29 Nonetheless, in this research SCC was more prevalent among HPV 18 infected patients. Prevalence studies have shown that 60–70% of SCC cases occurred in HPV16 or HPV18 infected women.30
In this study, there was no statistically significant association between HPV presence and sociodemographic variables in women with cervical cancer. A similar result was obtained by Chen et al. (2015), who sought to analyze the distribution of HPV 18 variants according to histological type (SCC or ADC). HPV 18 sublineages were compared in 453 cases of cancer and 236 controls, as well as in 81 cases of ADC and 160 cases of SCC. However, no statistically significant difference was found in the distribution of HPV 18 variants between cases and controls or between ADC and SCC at both general and regional level.11 The study of Arias-Pulido et al. (2005) with 15 cases of ADC and 10 of SCC have not found statistically significant differences in the distribution of HPV 18 variants among the patients analyzed. These results indicate that the high prevalence of HPV 18 in ADC is due to the specific tropism of HPV 18 variants for glandular cells.11,31,32
Strengths and limitationsThe limiting factor in this study was the low number of women infected with HPV 18 and the lack of a control group to compare women with HPV 18 and cervical cancer and those with HPV 18 but without cervical cancer. This was the first study conducted in the Northeast of Brazil to analyze the distribution of HPV 18 variants in women with cervical cancer who were not vaccinated against HPV. This population is resident in one of the regions with lowest socioeconomic and educational levels, with difficult access to health and education services.
ConclusionsIn conclusion, the results showed that women with cervical cancer are associated with a higher frequency of HPV 16 and 18 in the Northeast region of Brazil, and with a high prevalence of lineage A among women infected with HPV 18. Information about the diversity of HPV may contribute to the development of diagnostic and therapeutic approaches in order to reduce the number of cervical cancers.
The data from this study provide important information regarding the distribution of HPV in this population and will provide a basis for future studies on the distribution of HPV after the implementation of HPV vaccination and how it impacts on the number of cervical cancer cases in Brazil.
List of abbreviationsADC, adenocarcinoma; HPV, human papillomavirus; ICF, Informed Consent Form; SCC, squamous cell carcinoma; SNV, single-nucleotide variations; PCR, polymerase chain reaction; UMT, undifferentiated malignant tumor.
Ethics approval and consent to participateThis project was approved by the Research Ethics Committee of the Federal University of Maranhão CEP-UFMA), under Consolidated Opinion N° 1.289.419/2015. Women that accepted to participate by signing an Informed Consent Form.
Consent for publicationWomen that accepted to participate by signing an Informed Consent Form, giving their consent for publication.
Availability of data and materialsAll data generated or analysed during this study are included in this published article.
FundingThis research was supported by Fundação de Amparo à Pesquisa e ao Desenvolvimento Científico e Tecnológico do Estado do Maranhão (FAPEMA) (BEPP 10/2015).
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Authors' contributionsGRBS, APAC, ZSB, FCBV and MDSBN participated in interpretation of data, drafted and critically revised the manuscript. RSL, FVF, ZSB, LMOB, MCB, ECF, MAMM and MDSBN contributed to study design, interpretation of data, and critically revised the manuscript. MACNS, APAC, LHLC and FRBS analyzed and assisted in interpretation of the data and assisted in drafting the manuscript. WEMF, DFS, MCB and ECF contributed to interpretation of data and critically revised the manuscript. All authors read and approved the final manuscript
We thank to Dr. Miguel Ângelo Martins Moreira from the National Institute of Cancer, Genetic Division for the HPV variants analyses.
We thank the Center for Advanced Studies of the State University of Caxias (UEMA) for the HPV genotyping.
We thank to Dr. Rodrigo Lopes for specimens collect.
We thank to CAPES – Finance Code 001.