The widespread use of antiretroviral therapy increased the transmission of antiretroviral resistant HIV strains. Antiretroviral therapy initiation during acute/recent HIV infection limits HIV reservoirs and improves immune response in HIV infected individuals. Transmitted drug resistance may jeopardize the early goals of early antiretroviral treatment among acute/recent HIV infected patients.
MethodsPatients with acute/recent HIV infection who underwent resistance test before antiretroviral treatment initiation were included in this analysis. HIV-1 sequences were obtained using an in house protease/reverse transcriptase genotyping assay. Transmitted drug resistance was identified according to the Stanford HIV Database for Transmitted Drug Resistance Mutations, based on WHO 2009 surveillance list, and HIV-1 subtyping according to Rega HIV-1 subtyping tool. Comparison between patients with and without transmitted drug resistance was made using Kruskal–Wallis and Chi-square tests.
ResultsForty-three patients were included, 13 with acute HIV infection and 30 with recent HIV infection. The overall transmitted drug resistance prevalence was 16.3% (95% confidence interval [CI]: 8.1–30.0%). The highest prevalence of resistance (11.6%, 95% CI: 8.1–24.5) was against non-nucleoside reverse transcriptase inhibitors, and K103N was the most frequently identified mutation.
ConclusionsThe high prevalence of nonnucleoside reverse transcriptase inhibitors resistance indicates that efavirenz-based regimen without prior resistance testing is not ideal for acutely/recently HIV-infected individuals in our setting. In this context, the recent proposal of including integrase inhibitors as a first line regimen in Brazil could be an advantage for the treatment of newly HIV infected individuals. However, it also poses a new challenge, since integrase resistance test is not routinely performed for antiretroviral naive individuals. Further studies on transmitted drug resistance among acutely/recently HIV-infected are needed to inform the predictors of transmitted resistance and the antiretroviral therapy outcomes among these population.
The widespread usage of antiretroviral therapy (ART) and the increased survival of individuals using it favor the transmission of resistant HIV strains. Transmitted drug resistance (TDR) may be higher among patients with acute infection than in patients with chronic HIV infection.1,2 This may lead to a more rapid decline in CD4 cell counts prior to ART initiation and limit both the magnitude and duration of treatment response.3–7 TDR testing during acute HIV infection (AHI) provides increased sensitivity for the detection of primary drug resistance even before the overgrowth of drug-sensitive viral quasi-species.8
Early ART initiation during acute and recent HIV infection have benefits in limiting HIV reservoirs and improving immune response9,10 if a full active ART regimen is promptly initiated. TDR may affect the time for ART response (virologic clearance) and jeopardize the early treatment goals among acute/early HIV infected individuals.
Currently, TDR testing is not standardized in most resource-limited settings, including Brazil. However, TDR surveillance is needed to assess the emergence and spread of drug-resistant strains in order to inform HIV treatment guidelines.
The HIV epidemic in Brazil persists concentrated and unabated among men who have sex with men (MSM), with a high proportion of them remaining unaware of their HIV status.11 Rio de Janeiro is one of the major epicenters of the HIV epidemic in Brazil, contributing with 79,078 AIDS cases from 2000 to July 2015, holding the second position in number of cases within the country.12
We hereby report the prevalence of TDR and drug mutations associated with resistance in a cohort of acutely/recently HIV-infected individuals in Rio de Janeiro, Brazil, majority of whom MSM, to assess the need for routine TDR surveillance in Brazil.
MethodsThe Instituto Nacional de Infectologia Evandro Chagas – Fiocruz (INI) is the largest provider of primary, specialty, and tertiary care for individuals living with HIV/AIDS in Rio de Janeiro, Brazil. A clinical cohort has been maintained since 1986 and cohort procedures have been described elsewhere.13 Since August 2013, we have been enrolling individuals with acute and recent HIV infection and offering them immediate ART, with the goal of reducing inflammation and HIV reservoirs.
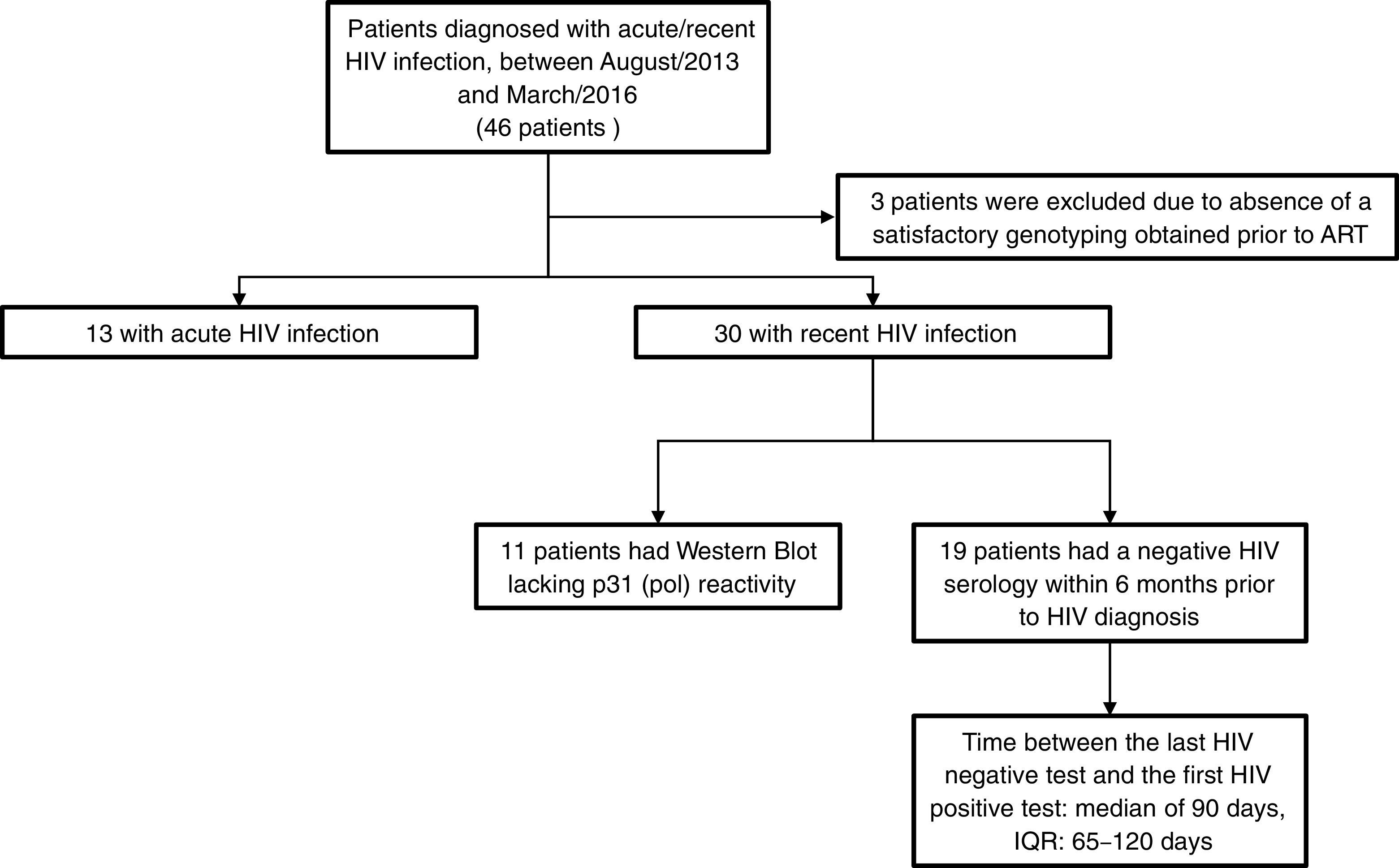
For this analysis, we included 46 patients who were diagnosed with acute/recent HIV infection, between August/2013 and March/2016. Inclusion criteria were age over 18 years, documented seroconversion within the previous six months and no prior ART. HIV drug resistance testing was performed using an in-house protease/reverse transcriptase genotyping assay developed by FIOCRUZ,14 which is certified by the National Institute of Allergy and Infectious Diseases virology quality assessment (NIAID-VQA). Drug resistance mutations (DRMs) were identified through the Stanford HIV Database for Transmitted DRM (TDRM/CPR Tool) Code Version 6.015 on the 2009 World Health Organization surveillance of transmitted DRMs list.16 HIV-1 subtyping was obtained by using REGA HIV-1 & 2 Automated Subtyping Tool (Version 2.0).17 Acute HIV infection (AHI) was defined as a negative result for a third generation HIV rapid test followed by a reactive result for the HIV antigen/antibody combination assay, or a detectable HIV RNA testing on pooled and subsequently confirmed with an individual HIV RNA test. Recent HIV infection (RHI) was defined as a reactive HIV serology and a documented HIV negative serology within the prior six months or a reactive Western Blot lacking p31 (pol) reactivity. Between-groups comparisons were made using Kruskal–Wallis test and Chi-square tests for continuous and categorical variables, respectively.
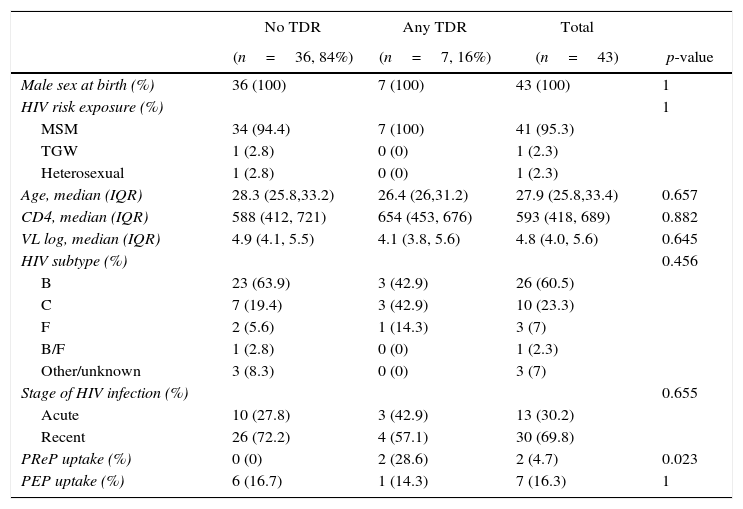
ResultsOut of the 46 included patients, 43 had a satisfactory protease/reverse transcriptase HIV-1 genotyping obtained prior to ART initiation and, of them 13 (30.2%) were defined as AHI and 30 (69.8%) as RHI (Fig. 1). The median time between the genotypic resistance test and HIV diagnosis was seven days (interquartile range [IQR]: 2–21 days). All patients were male at birth (one transgender woman), 95% reported having sex with men (Table 1). Median age at HIV diagnosis was 28 years old (IQR: 26–33 years), median CD4 and HIV RNA were 593cells/mm3 (IQR: 418–689cells/mm3) and 4.8 log (IQR: 4.0–5.6 log), respectively. The most frequent HIV subtype was B (60.5%), followed by subtypes C (23.3%) and F (7%). No significant differences in socio-demographic and clinical variables were observed between patients with and without DRM (Table 1).
Patient characteristics and prevalence of transmitted drug resistance according to the WHO SDRM 2009 list.
| No TDR | Any TDR | Total | ||
|---|---|---|---|---|
| (n=36, 84%) | (n=7, 16%) | (n=43) | p-value | |
| Male sex at birth (%) | 36 (100) | 7 (100) | 43 (100) | 1 |
| HIV risk exposure (%) | 1 | |||
| MSM | 34 (94.4) | 7 (100) | 41 (95.3) | |
| TGW | 1 (2.8) | 0 (0) | 1 (2.3) | |
| Heterosexual | 1 (2.8) | 0 (0) | 1 (2.3) | |
| Age, median (IQR) | 28.3 (25.8,33.2) | 26.4 (26,31.2) | 27.9 (25.8,33.4) | 0.657 |
| CD4, median (IQR) | 588 (412, 721) | 654 (453, 676) | 593 (418, 689) | 0.882 |
| VL log, median (IQR) | 4.9 (4.1, 5.5) | 4.1 (3.8, 5.6) | 4.8 (4.0, 5.6) | 0.645 |
| HIV subtype (%) | 0.456 | |||
| B | 23 (63.9) | 3 (42.9) | 26 (60.5) | |
| C | 7 (19.4) | 3 (42.9) | 10 (23.3) | |
| F | 2 (5.6) | 1 (14.3) | 3 (7) | |
| B/F | 1 (2.8) | 0 (0) | 1 (2.3) | |
| Other/unknown | 3 (8.3) | 0 (0) | 3 (7) | |
| Stage of HIV infection (%) | 0.655 | |||
| Acute | 10 (27.8) | 3 (42.9) | 13 (30.2) | |
| Recent | 26 (72.2) | 4 (57.1) | 30 (69.8) | |
| PReP uptake (%) | 0 (0) | 2 (28.6) | 2 (4.7) | 0.023 |
| PEP uptake (%) | 6 (16.7) | 1 (14.3) | 7 (16.3) | 1 |
TDR, transmitted drug resistance; MSM, men who have sex with men; TGW, transgender women; VL, HIV RNA viral load; PReP, pre-exposure prophylaxis; PEP, post-exposure prophylaxis.
The overall TDR prevalence was 16.3% (95% confidence interval [CI]: 8.1–30.0%), being 23.1% (95% CI: 8.2–50.3%) among those diagnosed with AHI and 13.3% (95% CI: 5.3–29.7%) among those with RHI. Overall, five patients presented non-nucleoside reverse transcriptase inhibitors (NNRTI) DRMs, yielding a prevalence 11.6% (95% CI: 5.1–24.5%), and K103N was the most frequently identified resistance mutation (three patients). The other NNRTI DRMs were K101E and G190A (one patient each). Two patients presented protease inhibitors (PI) DRMs (prevalence of 4.7%, 95% CI: 1.3–15.5%) (I47A, I85V), whereas only one presented nucleoside reverse transcriptase inhibitors (NRTI) DRMs (prevalence of 2.3%, 95% CI: 0.4–12.1%, all thymidine analog mutations [TAMs], including M41L, D67N, T215S/C, K219Q/E). No triple-class TDR was identified.
Of note, two individuals were exposed to pre-exposure prophylaxis (PrEP, oral daily tenofovir plus emtricitabine) before HIV diagnosis. One of them, defined as AHI, started PrEP 175 days before seroconversion and the genotypic test revealed only a PI DRM (I47A). The other patient, with RHI, had interrupted PrEP use 140 days before seroconversion (after almost one year on PrEP), and at baseline presented DRMs for both NRTI (TAMS: M41L, D67N, T215S/C, K219Q) and NNRTI (G190A). Neither of them presented emtricitabine or tenofovir DRM.
Seven patients used post-exposure prophylaxis (PEP) prior to HIV diagnosis and only one of them presented with a DRM (K103N), which was not related to the ARV used as PEP (PI based regimen). Two patients were on PEP at the moment of AHI diagnosis, both diagnosed with detectable HIV viral load and negative HIV rapid test and with no DRM at baseline. One of them had been using tenofovir, lamivudine and zidovudine (as PEP regimen) for four days prior to HIV diagnosis, with a subsequent switch to tenofovir, lamivudine and efavirenz. The second patient had been using zidovudine, lamivudine and lopinavir/ritonavir for eight days prior to HIV diagnosis and, switching thereafter to tenofovir, lamivudine, lopinavir/ritonavir, and raltegravir.
DiscussionHerein, we observed a TDR prevalence rate of 16.3% among acutely/recently HIV-infected individuals, in Rio de Janeiro, Brazil. This prevalence is within the range observed among AHI/RHI in other settings in samples collected between 1995 to 2013 (8.3–21%),1,8,18–24 with similar rates detected in Brazilian studies with samples collected between 1996 and 2012 (8.0–32%).25 This rate was higher than those found in chronically HIV-infected individuals in Brazil, which varied from 3.6% to 11%.26,27 Most studies addressing TDR enroll chronically HIV-infected participants28 (after the overgrowth of more fit, drug-sensitive viral quasi-species) which limits the sensitiveness of TDR detection.
The prevalence of NNRTI DRM found in the present study (11.6%) is higher than that observed among acute HIV infected individuals in Thailand (5.0%)19 and in acute/recent HIV infected individual from China (1.7%).20 Of note, NNRTI DRM prevalence was even higher than the rate previously reported by our group (6.3%)29 in HIV recently infected individuals from Rio de Janeiro between 2005 and 2007 (classified using BED capture enzyme immunoassay).
The duration of ART availability has been an important predictor of TDR emergence.30 Universal access to ART as well as treatment monitoring is available in Brazil since 1997. Brazilian treatment guidelines recommend NNRTI based regimens as first line ART since 2000, earlier than the ART scale up in other low/middle-income countries,19,20 and this may have negatively impacted the emergence of NNRTI TDR in our setting.
Brazil has a concentrated epidemic among MSM, transgender women, female sex workers, and drug users.31 While HIV prevalence among the general population is 0.6%, in MSM it reaches 14.2%.11 Young MSM currently account for nearly 40% of the AIDS cases in the country, with increases of 41.3% (aged 15–19 years) and 25.1% (aged 20–24 years) observed from 2004 to 2013.12 Targeting high-risk populations, early ART initiation and implementing TDR surveillance are utmost important strategies to control HIV epidemic.
In addition, acutely infected individuals account for 25–50% of the HIV transmission.32,33 Transmission rates are sharply elevated during the first three months of HIV infection,34 likely due to increased viral concentrations35 and founder virus transmission advantages that facilitate transmission at a lower inoculum.36 Phylogenetic analyses among MSM suggest large early-stage contributions,37,38 posing a high risk of TDR spread through unprotected anal intercourse, as observed among young Thai MSM.19
Although highly effective, NNRTI regimens have low genetic barrier. Considering the high prevalence of NNRTI TDR observed in our patient sample, the use of efavirenz-based regimens without prior resistance testing might result in high rates of early virologic failure. Prompt initiation of fully active ART during acute/recent HIV infection can limit HIV reservoir seeding/size, improve immune response,2 and reduce HIV transmission.39 TDR may hamper virologic clearance; thus, jeopardize early treatment benefits in those individuals. Pre-treatment genotyping is of utmost importance to reach the goals of early treatment among acute/recent HIV infected individuals. Besides, pre-treatment genotyping (regardless of HIV infection stage) has been shown to be cost-effective in the Brazilian context.40 Nevertheless, so far, it is solely recommended for pregnant women, vertically infected newborns, and individuals who were infected by a known HIV-infected partner under ART.41
Concerns have been raised regarding the increased risk of TDR42 with the expansion of PrEP and PEP43 uptake among high-risk populations. Of note, in our cohort PrEP and PEP users did not present any drug resistance mutation related to their regimen, which is in agreement with recent evidence from iPrEx.44 Given the small number of individuals exposed to PrEP/PEP prior to seroconversion in our study, these results should be taken cautiously. Continuous surveillance will be needed to monitor resistance with the expansion of these strategies.
There are several limitations that need to be highlighted in the present study, some of which were already addressed throughout this discussion. First, the comparison of DRM prevalence by drug classes (i.e. NNRTI vs. PI) in our setting with those reported in the United States and other high-income settings must be done cautiously since the differences in first line ART regimen can influence this prevalence. Second, our study used a convenience sample from a single cohort in Rio de Janeiro; hence our results may not reflect the reality of other HIV populations in Brazil. And finally, our TDR prevalence may be underestimated considering that our resistance tests results were based on standard genotypic testing using Sanger sequencing that is the methodology we have being using for the routine care as well as in other research studies. Future studies implementing assays such as ultra-deep sequencing and allele-specific PCR are in need in order to evaluate TDR rates using tests that are more sensitive.
In conclusion, TDR surveillance among acutely/recently HIV-infected individuals can provide critical data to guide antiretroviral regimen choice. Notwithstanding our small and non-probabilistic sample, the results indicate that a NNRTI based regimen without prior resistance testing is not ideal for acutely/recently HIV-infected individuals in our setting. PI or integrase inhibitors (INSTIs) based regimens may be safer in order to avert onward TDR while preserving the benefits of early effective ART. In this context, the recent proposal of including an integrase inhibitor as first line ART regimen in Brazil could be an advantage for treating newly HIV infected individuals. However, it also poses a new challenge, since the integrase sequencing for resistance test is not routinely performed for ART naive individuals. Future research addressing TDR on acutely/recently HIV-infected are needed to provide information on predictors of TDR and ART outcomes in this population.
Conflicts of interestThe authors declare no conflicts of interest.
Beatriz Grinsztejn acknowledges the funding from the National Council of Technological and Scientific Development (CNPq) and the Research Funding Agency of the State of Rio de Janeiro (FAPERJ).