Viral conjunctivitis are the most frequent infections in ophthalmology clinics. The diagnosis is usually relying on clinical findings and medical history. However, topical antibiotics are often used unnecessarily addition to symptomatic treatment because of unsure agents. We aimed to detect the Adenovirus, Coxsackievirus and Enterovirus from conjunctiva and pharyngeal samples of patients.
MethodsThe conjunctiva and pharyngeal samples of the patients with conjunctivitis were taken by Virocult transport media and kept at −80¿C up to study day. Adenovirus spp, Enterovirus 70 and Enterovirus 71, Coxsackie A24 and Coxsackie A16 were detected by real-time PCR. Samples from healthy health care workers of ophthalmology clinic were used for control group.
ResultsA total of 176 samples (conjunctival and pharyngeal samples of 62 patient and 26 healthy subjects) were included. The mean age of 34 (55.7%) male and 27 (44.3%) female patients was 34±17. Twenty five (40.3%) of the patients were receiving antibiotic drops at first visit. The main etiologic agent in conjunctival samples was found to be Adenovirus (46/62, 74.2%) followed by Enterovirus 70 (4/62, 6.4%) and Enterovirus 71 (4/62, 6.4%). Coxsackievirus 16 and 24 were also found in 2 patients (1/62 each, 1.6%). Pharyngeal samples were also positive for Adenovirus (20/62, 32.3%), Enterovirus 70 and 71 (7/62, 11.3% and 5/62, 8.1% respectively), Coxsackievirus 16 and 24 (2/62, 3.2% and 1/61, 1.6%).
ConclusionsIt is very difficult in viral conjunctivitis to make clinical differentiation caused by different agents because of common clinical signs and symptoms. In routine clinical work, the viral conjunctivitis usually related with Adenovirus. But almost one fourth of the patients’ conjunctivitis were not related to Adenovirus, which shows the importance of the laboratory diagnostics. True diagnosis plays an important role on prevention of contamination and unnecessary use of antibiotics in viral conjunctivitis.
Adenoviruses, a major cause of viral conjunctivitis,1–3 are double-stranded non-enveloped DNA viruses belonging to the family Adenoviridae, genus Mastadenovirus. Fifty-one serotypes of human adenoviruses have been recognized and classified into seven species (A-G) based on genome sequencing, phylogenetic, and biological characteristics.4 Adenoviruses are implicated in a wide range of human diseases including pharyngoconjunctival fever, an acute and highly infectious disease characterized by fever, pharyngitis, acute follicular conjunctivitis, and regional lymphoid hyperplasia with tender, enlarged preauricular adenopathy.1,3 The serotypes 3, 4, and 7 are frequently associated with the pharyngoconjunctival fever. Epidemic keratoconjunctivitis (EKC) is a highly contagious, severe form of conjunctivitis, which may lead to outbreaks worldwide. Adenovirus serotypes 8, 11, 19, and 37 are common etiologic agents of epidemic keratoconjunctivitis with severe symptoms, such as severe discharge, lacrimation, membrane formation, and multiple subepithelial corneal infiltrates. Acute nonspecific follicular conjunctivitis is mostly caused by human adenovirus serotype 3, 4, 7, and 14, but it does not involve the cornea and the resulting conjunctivitis is typically mild. Nonspecific follicular conjunctivitis usually resolves within a week to 10 days without treatment. Chronic keratoconjunctivitis is the rarest form of ocular adenoviral infection and is caused by adenovirus type 2, 3, 4, and 53.3,4 Acute hemorrhagic conjunctivitis (AHC) is an epidemic form of highly contagious conjunctivitis mostly caused by Enterovirus 70 and Coxsackievirus A24 variant.3,5 Herpes simplex virus, rubella, rubeola, varicella zoster, Epstein–Barr and Newcastle viruses are the other agents for viral conjunctivitis.6
The diagnosis of viral conjunctivitis is usually performed on the basis of patient history and clinical findings, although serologic and molecular laboratory diagnoses are also currently available. Unnecessary antibiotic therapy is frequently administered as a result of unknown etiology. The aim of this study was to identify the most common etiologic agents of acute conjunctivitis and determine the relationship between the etiologic agents and the clinical findings, complications, and systemic findings among acute conjunctivitis cases.
MethodsClinical definitionA prospective, controlled clinical study was conducted to evaluate 62 consecutively enrolled patients that were admitted to the Ankara Atatürk Training and Research Hospital between July 2013 and September 2014 with suspected viral conjunctivitis (who had at least one of the following complaints and findings: hyperemia, lacrimation, foreign body sensation, discharge, burning, follicular conjunctivitis, conjunctival hemorrhages, membrane formation, eyelid swelling, or conjunctival hemorrhages) or keratoconjunctivitis (those who had punctate corneal defects or subepithelial infiltrates in addition to viral conjunctivitis findings). Twenty-six healthy volunteers working in the ophthalmology department at all times were included as controls.
This study followed the tenets of the Declaration of Helsinki and was approved by the local ethics committee. Written informed consent was obtained from the study participants before sample collection.
Sample collectionPharyngeal and conjunctival samples were collected from patients and controls by two experienced ophthalmologists. The samples were placed in viral transport medium (Virocult, BD) with swabs and stored at −80°C until analysis.
Sample processing and virus identificationMolecular identification was used for Enterovirus 70/71. Coxsackievirus A16/A24v and adenovirus were determined using a real-time PCR kit (DaAn Gene Co., Ltd, Guangzhou, China) using virus-specific primers and fluorescein labeled probes. Viral DNA and RNA extraction were performed in a 200μl sample volume using PureLink Viral DNA and RNA kit (Invitrogene, Thermo Fisher Scientific, Waltham, MA, USA) according to the manufacturer's instructions. After elution of viral DNA and RNA samples in a 50μl elution buffer, the concentration of DNA/RNA was confirmed with Qubit® 2.0 Fluorometer (Invitrogene, Thermo Fisher Scientific, Waltham, MA, USA). The real-time PCR conditions for Enterovirus 70/71, Coxsackievirus A16/A24v, and Adenovirus were carried out according to the manufacturer's instructions. Adenovirus reaction was performed in a total 45μl reaction volume using 2μl sample supernatant, 3μl of Taq mixture, and 40μl of ADV PCR reaction solution. The real-time PCR reaction conditions for ADV was one cycle of 93°C for 2min, 10 cycles of 93°C for 45s and 55°C for 1min, and 30 cycles of 93°C for 30s and 55°C for 45s. The real-time PCR reactions for Enterovirus 70/71 and Coxsackievirus A16/A24v were set at 17μl of PCR reaction solution A, 3μl of PCR reaction solution B, and 5μl of sample supernatant. The real-time PCR reaction conditions for Enterovirus 70/71 and Coxsackievirus A16/A24v were one cycle of 50°C for 15min, one cycle of 95°C for 15min and 40 cycles of 94°C for 15s and 55°C for 45s. All real-time PCR reactions were run with negative and positive controls. Data collection in all the reactions was set as step 2 (55°C) of stage 3. The Cycle Threshold Value (Ct) above 30 and 38 was accepted as negative for Adenovirus and Enterovirus 70/71, and Coxsackievirus A16/A24v, respectively. All real-time PCR reactions were performed using Applied Biosystems, 7500 Fast Real-Time PCR System (Applied Biosystems, Thermo Fisher Scientific, Waltham, MA, USA).
All Statistical analysis was performed using SPSS 12.0 software (SPSS Inc., Chicago, IL, USA).
ResultsA total of 124 samples (62 conjunctiva and 62 pharynx) from 62 patients were collected. The mean age was 34 years for male (54.8%) and 28 for female (45.2%) patients; the overall mean age was 34±17. Fifty-two samples (26 conjunctiva and 26 pharynx) from 26 healthy controls were also collected. The mean age was 8 years for male (30.8%) and 18 for female (69.2%) controls; the overall mean age was 39±10 years. Twenty-five (40.3%) patients received antibiotic drops at the first visit.
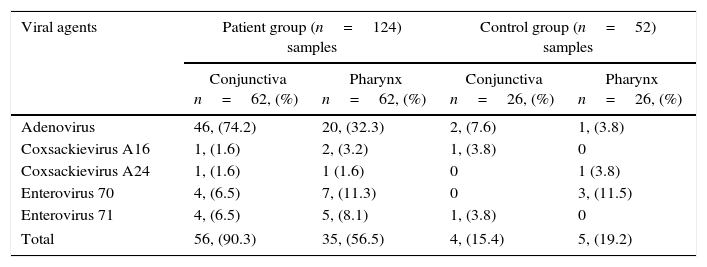
Among the 62 patients with acute conjunctivitis, 50 (80.6%) had conjunctiva samples with PCR positive for one of the viral agents. Both conjunctival and pharyngeal samples in 25 patients (40.3%) were PCR positive for one of the viral agents. Conjunctival adenovirus isolates correlated significantly with pharyngeal adenovirus isolates (r=0.407, p=0.01) (Table 1).
Viral agents isolated from the patient and the control groups.
| Viral agents | Patient group (n=124) samples | Control group (n=52) samples | ||
|---|---|---|---|---|
| Conjunctiva n=62, (%) | Pharynx n=62, (%) | Conjunctiva n=26, (%) | Pharynx n=26, (%) | |
| Adenovirus | 46, (74.2) | 20, (32.3) | 2, (7.6) | 1, (3.8) |
| Coxsackievirus A16 | 1, (1.6) | 2, (3.2) | 1, (3.8) | 0 |
| Coxsackievirus A24 | 1, (1.6) | 1 (1.6) | 0 | 1 (3.8) |
| Enterovirus 70 | 4, (6.5) | 7, (11.3) | 0 | 3, (11.5) |
| Enterovirus 71 | 4, (6.5) | 5, (8.1) | 1, (3.8) | 0 |
| Total | 56, (90.3) | 35, (56.5) | 4, (15.4) | 5, (19.2) |
Conjunctival samples of six patients were positive for mixed infections. Adenovirus was isolated from three (50%) of the six Enterovirus 70 isolated from conjunctiva samples; two (33.3%) of the six Enterovirus 71 isolated from conjunctiva samples, and one (16.7%) of the six Coxsackievirus A16 isolated from conjunctiva samples.
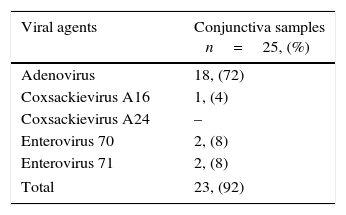
The clinical presentation was bilateral in 32.2% (20/62) of the patients. The most common symptoms were hyperemia, lacrimation, foreign body sensation, discharge, and burning, in decreasing order. Follicular conjunctivitis (n=54/64, 84.3%), eyelid swelling (n=36/62, 58.1%), conjunctival hemorrhages (n=9/62, 14.5%), membrane formation (n=6/62, 9.7%), punctate corneal defects (n=4/62, 6.4%), and subepithelial infiltrates (n=2/62, 3.2%) were the most reported findings in the patient group. The prevailing extraocular symptoms were lymphadenopathy (14/62, 22.6%) and systemic symptoms like headache and fever (12/62, 19.4%). Pharyngitis was also observed in 16.1% (10/62) of the patients. Seventy-six percent of the patients who had already received antibiotic drops at their first visit had PCR positive conjunctival specimens. In this group, as well as in the total study group, the detected viral agents were Adenovirus (18/25, 72%), Enterovirus 70 (2/25, 8%), Enterovirus 71 (2/25, 8%), and Coxsackievirus 16 (1/25, 4%), in decreasing order. Coxsackievirus 24 was not detected in this group (Table 2).
There was no significant correlation between viral agents and clinical findings except for adenoviruses. Conjunctival adenovirus was isolated from all patients with conjunctival membranes, 75% of patients with punctate staining, and 92.8% of patients with lymphadenopathy. The mean symptomatic duration of the disease was 26.42 days. However, the duration was 23.3 days for the patients who had received antibiotics at the first visit. Although antibiotic drops reduced the duration of the disease, the difference was not significant. Six patients (9.7%) continued to suffer from blurred visual acuity even after recovery from active infection. Two cases who continued to suffer from blurred visual acuity even after recovery from active infection were the ones with punctate corneal epithelial defects at initial presentation.
DiscussionAcute conjunctivitis is a rather common disease, which may affect many people and impose economic and social burdens. Studies have shown that viruses cause up to 35–80% of all cases of acute conjunctivitis2,7,8 and between 65% and 90% of cases of viral conjunctivitis are caused by adenoviruses.1,3,5 In line with these studies, in our investigation Adenovirus was the most commonly isolated causative agent of acute conjunctivitis from both conjunctiva and pharynx samples (74.2% from conjunctiva samples, 32.3% from pharynx samples).
The second most frequently observed causative agents were Enterovirus 70 and Enterovirus 71, respectively. Previously, Li et al. isolated Coxsackievirus A24 as the second most common viral agent following adenoviruses for acute conjunctivitis, but no Enterovirus 70 was isolated. Additionally, adenoviruses were the most frequently identified agents in co-infections of acute conjunctivitis, which is concordant with our data.5
Viral conjunctivitis, secondary to adenoviruses, is highly contagious, and the virus spreads through direct contact via contaminated fingers, medical instruments, swimming pool water, or personal items. Hand washing and isolation of the infected patients are essential to avoid transmission.9 None of the cases have a history of exposure to swimming pool water. In 35% of the cases, the contamination was intrafamilial transmission through hands and personal items. No clinicians involved in sample collection or in treating the patients were contaminated because proper precautions were taken.
Redness, itching, burning, watery discharge, foreign body sensation, follicular conjunctivitis, membrane formation, lymphadenopathy, and hemorrhages are common symptoms in viral conjunctivitis.3,5 Our findings are in accordance with the literature, as the same common symptoms were observed, making clinical diagnoses much easier. In this study, some symptoms were significantly associated with adenoviruses (p<0.05). For example, conjunctival adenovirus was isolated from all patients with conjunctival membranes, 92.8% of the patients with lymphadenopathy, and 75% of the patients with punctate staining. However, there were no significant correlations among other symptoms and viral agents.
The diagnosis of the viral conjunctivitis is usually made on the basis of patient history and clinical findings. Viral cultures by conventional techniques are the gold standard, but may be insensitive for certain samples and take up to 21 days to develop the cytopathic effect.4 PCR is a useful technique that amplifies small amounts of viral DNA with great sensitivity and specificity. A laboratory confirmation of the virus-related etiology might aid the physician in making an accurate diagnosis and taking hygienic precautions, and therefore reduce the spread of the disease.4,10 In this study, we collected both conjunctiva and pharynx samples from patients with acute conjunctivitis and used the PCR method for identification of viral agents. We primarily observed among those patients with varying demographic characteristics and diagnosed with acute conjunctivitis that the most commonly detected agents were viruses. Of the 62 acute conjunctivitis cases, 80.6% of the conjunctiva samples were PCR positive for one of the viral agents. In addition, in 40.3% of the patients, both pharyngeal and conjunctival samples yielded the same viral agent.
Because of overlapping features in clinical presentation, definitive diagnosis of infectious conjunctivitis can be challenging.5 The result of this study revealed that although there were differences in clinical presentation, there were also some overlaps. Previously, Marangon et al. reported a significant correlation between laboratory and clinical findings in viral diseases.11
Our isolation of infectious viral agents from the samples of asymptomatic controls who worked in ophthalmology clinics indicates contamination from the patients.
In the study of Li et al., single infections were observed in 49.89% of cases and mixed infections were detected in 2.36%.5 In our study, conjunctival samples of six patients (9.5%) were positive for mixed infections. Adenovirus was isolated from conjunctiva samples together with Enterovirus 70 in three patients, with Enterovirus 71 in two patients, and in one patient together with Coxsackievirus A16. Among the patients who were infected with mixed viral agents, the reason for the male predominance and the age range of 25–35 may be due to the increased exposure time and frequency of contact with the source of infection.3,5
Although no effective treatment exists, artificial tears and cold compresses may relieve some of the symptoms.6 Antibiotic drops are not indicated for viral conjunctivitis as their use may complicate the clinical presentation by causing allergy and toxicity.3,12,13 Most of the medicines are prescribed inappropriately. Rational drug use requires five criteria including: accurate diagnosis, accurate prescription, accurate dispensation, suitable packaging, and patient orientation.14 Increased antibiotic resistance is also of concern with frequent and inappropriate use of antibiotics. Antibiotic resistance also occurs in the management of eye infections with antibiotic drops.15 Udeh et al. demonstrated that the correct identification of patients with viral conjunctivitis might reduce the costs related to inadequate use of antibiotics in patients with EKC.10 Inappropriate antibiotic drops and nonsteroidal anti-inflammatory drops may lead to histological and structural toxicity in conjunctiva.16,17 In addition, adenoviral conjunctivitis is associated with significant complications, including subepithelial infiltrates, lacrimal drainage abnormalities, and symblepharon formation.18
A rapid, inexpensive and accurate method for diagnosing adenoviral ocular infections is needed not only to limit the transmission of the virus within communities, but also to avoid the expensive, unnecessary, and ineffective use of antibiotic therapies.10
In this study, we investigated viral agents in conjunctivitis in a tertiary medical center in Turkey. The limitation of this study was the lack of genotyping of adenovirus. Adenovirus can give rise to epidemic outbreaks both in the general population and in hospital environments. The correct identification of patients with viral conjunctivitis may reduce the spread of the disease and limit unnecessary antibiotic treatments that may lead to economic burden and antibiotic resistance. Nationwide multicenter studies may yield more accurate data about the prevalence and etiology of viral conjunctivitis.
Conflicts of interestThe authors declare no conflicts of interest.