Chlamydia trachomatis (CT) and Neisseria gonorrhoeae (GC) cause infections in the female genital tract, increasing susceptibility to and infectiousness of HIV. The objectives of the present study were to determine the prevalence and associated factors of CT and GC infection among HIV-infected women in Brazil.
MethodsCross-sectional study conducted from March to December 2015, including HIV-infected women attending referral centers in nine states of Brazil, aged 18–49 years, nonpregnant. An interview was conducted including socio-demographic, epidemiological and clinical characteristics. After the interview, gynecological examination was conducted to collect cervical cytology and vaginal secretion to C. trachomatis and N. gonorrhoeae tests through molecular biology.
ResultsA total of 802 (89.1%) women participated. The prevalence of CT was 2.1% (17/802) and CG was 0.9% (7/802). The prevalence of a positive test for both CT and/or GC was 2.7%. The factors associated with positive CT/GC test in the multivariate logistic regression analysis were abnormal Papanicolau smear (OR 4.1; 95% CI: 1.54–11.09) and the presence of abnormal cervical discharge (OR 2.6; 95% CI: 1.02–6.71). Among 377 women who reported previous STI 245 (65.0%) reported using condom more frequently after being diagnosed. 62 (16.4%) discovered the STI after the partner told he was infected; 157 (41.6%) had STI symptoms and looked for care, and 158 (41.9%) discovered it in a routine consultation for another reason.
ConclusionsThe control of STI represents a unique opportunity to improve reproductive health of women living with HIV. STI diagnosis can change their behavior and reduce the sexual transmission of HIV and bacterial STI.
Chlamydia trachomatis (CT) and Neisseria gonorrhoeae (GC) screening can prevent health complications. Infection in the lower genital tract can result in upper genital tract complications, such as pelvic inflammatory diseases, ectopic pregnancy, chronic pelvic pain, and infertility in asymptomatic women, and transmission of infection during pregnancy and labor.1,2 They also increase susceptibility and infectiousness of HIV infection.3
In HIV-infected women, infection with CT or GC is an important biologic marker of behavior that may expose others to HIV. Furthermore, CT and GC are associated with increased cervico-vaginal HIV shedding that may increase HIV transmissibility.4 Identification of HIV-infected women with CT or GC can help target preventive interventions such as promoting safer sexual practices. Treatment of sexually transmitted infections (STI) also may impact heterosexual HIV transmission.5
Previous studies from Brazil reported prevalence rates of 3.0% of CT and 0.9% of GC among HIV-infected women in Rio de Janeiro6 and in Manaus a rate of CT of 4.3%.7 These results were not different from data reported in Zambia that found a prevalence rate of 1% of CT and 1.4% of GC in HIV-infected women.8,9
The aim of the current study was to determine the prevalence of and associated factors for CT and GC among HIV-infected women attending referral care centers for HIV/AIDS in Brazil.
MethodsA cross-sectional study was conducted among women living with HIV/AIDS who attended referral care centers for HIV/AIDS in nine different Brazilian states: Amazonas, Pernambuco, Bahia, Federal District, Espirito Santo, Rio de Janeiro, São Paulo, Paraná, and Rio Grande do Sul, distributed in the five geographical regions of Brazil, from March through December 2015.
Nonpregnant women aged 18–49 years, with a positive result for HIV infection, being cared for at the gynecology service linked to reference hospitals, who accepted to participate were invited to take part in the study.
A 20-min face-to-face interview was conducted using a standardized questionnaire (validated in a pilot study) that included socio-demographic characteristics (age, education, marital status, family income, place of residence); epidemiological (smoking, use of alcohol and illicit drugs, use of condoms, number of sexual partners, sexual practices) and clinical (vaginal discharge, previous STI, stage of infection with HIV, CD4 cell count and HIV viral load). A pilot study was conducted in a small number of women living with HIV/AIDS to evaluate the reliability and validity of the questionnaire.
After the interview, gynecological examination was carried out to collect cervical cytology and vaginal/cervical secretion to test for CT and GC through molecular biology. Samples were analyzed in an automated system for real time PCR (COBAS 4800 CT/NG – Roche Molecular Systems, Branchburg, NJ) for detection of CT and GC, as per the manufacturer's instructions at the Molecular Biology Laboratory of the Infectious Diseases Unit of the Federal University of Espírito Santo and São Marcos Laboratory, a ISO 9001:2000 (INMETRO) and UKAS (England) certified private laboratory in Vila Velha (ES). Endocervical samples were collected using swabs, PreservCyt transport medium and stored at 10°C until their transportation at low temperature to the reference laboratory, in a period of seven to 10 days.
Selection of the study sample took into account the proportion of AIDS cases from the five geographical regions reported to the AIDS National Information Surveillance System in 2010 (consolidated with the Information Surveillance System for Mortality, laboratory tests – CD4 counts and HIV viral load, and antiretroviral therapy). A total of 12,845 HIV-infected women were reported: 9.7% from the North; 20.1% from the Northeast; 38.6% from the Southeast; 25.2% from the South, and 6.4% from the Midwest region. Based on these criteria nine clinics were included: one in Northern region, two in the Northeast, three in the Southeast, two in the Southern region, and one in the Midwest region.
The sample size was calculated to estimate the prevalence of CT and GC in women living with HIV/AIDS, with a 95% confidence interval (CI) bilateral size of 0.5%. It was assumed as the lowest expected frequency 0.9% of N. gonorrhoeae in women living with HIV/AIDS6; accepting a variation of ±0.3% a number of 773 women were necessary. Allowing for a loss of 10%, a final sample of 850 women distributed proportionally in each clinic 95 women per clinic were to be included.
Data were analyzed using the SPSS – data entry statistical program (Statistical Package for the Social Sciences) version 17.0. A preliminary analysis was performed using exploratory techniques on the data, to check the distribution patterns and trends of the variables. Univariate analysis was then performed to check for the presence of association between the variables. Chi-square tests were used to compare proportions and Student's t tests and variance analysis were used for testing differences between mean values. Univariate and multivariate odds ratios (ORs) (adjusted for potential confounders) and 95% CIs were reported. Variables that were significant at p<0.15 in univariate analysis and known confounders (e.g., age and education) were considered in the multivariate analysis using a stepwise multiple logistic regression model.
This project was submitted to and approved by the Research Ethics Committee (#131107/2012) of Center for Health Sciences of the Federal University of Espírito Santo. All selected women were invited to take part voluntarily in the study and those who accepted signed a written consent form. Those who were diagnosed as being infected by CT or GC received treatment as recommended by the Brazilian Ministry of Health guidelines.
ResultsOut of 850 eligible women 802 (94.4%) accepted to participate in the study, from March to December 2015. Median age was 39 (IQR 34–46) years and median years of education was 9 (IQR6-11). The prevalence of CT was 2.1% (17/802) and of CG 0.9% (7/802). The prevalence of a positive test for CT and/or GC was 2.7% (22 cases).
The prevalence rates by geographical region were: North 2.6%; Northeast 2.6%; Midwest 1.2%; Southeast 3.5%, and South 2.4%. There was no statistically significant difference between the geographical regions.
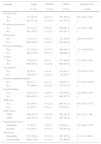
Table 1 shows demographic and behavior characteristics of women living with HIV in Brazil. None of the variables was associated to CT/GC positive test. A total of 43 (5.4%) women was younger than 25 years old, 299 (37.3%) had the first intercourse before 16 years, and 575 (71.7%) reported consistent condom use.
Demographic and behavior characteristics by CT/GC positivity among women living with HIV in Brazil, 2015 (N=802).
| Variable | Total | CT/GC+ | CT/GC− | OR (95% CI) |
|---|---|---|---|---|
| N (%) | N (%) | N (%) | p value | |
| Age (years) | ||||
| ≤24 | 43 (5.4) | 03 (7.0) | 40 (93.0) | 2.9 (0.83–10.28) |
| >24 | 759 (94.6) | 19 (2.5) | 740 (97.5) | 1 |
| Education (years) | ||||
| ≤8 | 398 (49.6) | 08 (2.1) | 390 (97.9) | 0.6 (0.24–1.38) |
| >8 | 404 (50.4) | 14 (3.5) | 390 (96.5) | 1 |
| Marital status | ||||
| Single/Divorced/Widow | 396 (49.4) | 12 (3.0) | 384 (97.0) | 1.2 (0.53–2.90) |
| Married/living together | 406 (50.6) | 10 (2.5) | 396 (97.5) | 1 |
| First sex intercourse | ||||
| ≤15 years | 299 (37.3) | 11 (3.7) | 288 (96.3) | 1.7 (0.73–3.99) |
| ≥16 years | 503 (62.7) | 11 (2.2) | 492 (97.8) | 1 |
| Number of partners (life) | ||||
| Only one | 67 (8.4) | 01 (1.5) | 66 (98.5) | 1 |
| 2–5 | 490 (61.1) | 15 (3.1) | 475 (96.9) | 1.0 (0.56–1.89) |
| 6–9 | 79 (9.9) | 02 (2.5) | 77 (97.5) | 1.2 (0.85–1.83) |
| ≥10 | 166 (20.7) | 04 (2.4) | 162 (97.6) | 1.4 (0.76–2.53) |
| Tobacco use | ||||
| Yes | 157 (19.6) | 06 (3.8) | 151 (96.2) | 1.6 (0.60–4.06) |
| No | 645 (80.4) | 16 (2.5) | 629 (97.5) | 1 |
| Illicit drug abuse | ||||
| Yes | 150 (18.7) | 6 (4.0) | 144 (96.0) | 1.7 (0.64–4.31) |
| No | 652 (81.3) | 16 (2.5) | 636 (97.5) | 1 |
| Injecting drug use | ||||
| Yes | 19 (2.4) | 01 (5.3) | 18 (94.7) | 2.0 (0.26–15.81) |
| No | 783 (97.3) | 21 (2.7) | 762 (97.3) | 1 |
| Consistent Condom use | ||||
| No | 227 (28.3) | 06 (2.6) | 221 (97.4) | 0.9 (0.36–2.45) |
| Yes | 575 (71.7) | 16 (2.8) | 559 (97.2) | 1 |
| Anal sex | ||||
| Yes | 350 (43.6) | 13 (3.7) | 337 (96.3) | 1.9 (0.80–4.50) |
| No | 452 (56.4) | 09 (2.0) | 443 (98.0) | 1 |
Clinical characteristics are described in Table 2. A total of 137 (17.1%) reported pelvic pain. Abnormal cervical discharge (5.7% vs. 1.7%, p=0.002); abnormal Papanicolau smear (9.9% vs. 2.1%, p<0.001), and detectable HIV viral load (4.7% vs. 2.0%, p=0.040) were associated with a positive CT/GC test result.
Clinical characteristics by CT/GC positivity among women living with HIV in Brazil, 2015 (N=802).
| Variable | Total | CT/GC+ | CT/GC− | OR (95% CI) |
|---|---|---|---|---|
| N (%) | N (%) | N (%) | p value | |
| Previous STI | ||||
| Yes | 377 (47.0) | 10 (2.7) | 367 (97.3) | 0.9 (0.40–2.20) |
| No | 425 (53.0) | 12 (2.8) | 413 (97.2) | 1 |
| Previous miscarriage | ||||
| Yes | 171 (21.3) | 5 (2.9) | 166 (97.1) | 1.1 (0.40–2.99) |
| No | 631 (78.7) | 17 (2.7) | 614 (97.3) | 1 |
| Pelvic pain | ||||
| Yes | 137 (17.1) | 6 (4.4) | 131 (95.6) | 1.9 (0.71–4.83) |
| No | 665 (82.9) | 16 (2.4) | 649 (97.6) | 1 |
| Cervical discharge | ||||
| Yes | 212 (26.4) | 12 (5.7) | 200 (94.3) | 3.5 (1.48–8.20) |
| No | 590 (73.6) | 10 (1.7) | 580 (98.3) | 1 |
| Cystitis | ||||
| Yes | 116 (14.5) | 4 (3.4) | 112 (96.6) | 1.3 (0.44–3.98) |
| No | 686 (85.5) | 18 (2.6) | 668 (97.4) | 1 |
| Genital ulcer | ||||
| Yes | 52 (6.5) | 1 (1.9) | 51 (98.1) | 1.5 (0.19–11/14) |
| No | 750 (93.5) | 21 (2.8) | 729 (97.2) | 1 |
| Genital Lymphadenopathy | ||||
| Yes | 26 (3.2) | 2 (7.7) | 24 (92.3) | 3.2 (0.70–14.29) |
| No | 776 (96.8) | 20 (2.6) | 756 (97.4) | 1 |
| Genital Itching | ||||
| Yes | 142 (17.7) | 7 (4.9) | 135 (95.1) | 2.2 (0.89–5.59) |
| No | 660 (82.3) | 15 (2.3) | 645 (97.7) | 1 |
| TARV use | ||||
| Yes | 718 (89.5) | 20 (2.8) | 698 (97.2) | 0.9 (0.20–3.70) |
| No | 84 (10.5) | 2 (2.4) | 82 (97.6) | 1 |
| CD4 Count | ||||
| ≥500 | 508 (63.3) | 15 (3.0) | 493 (97.0) | 0.8 (0.32–1.99) |
| ≤499 | 294 (36.7) | 7 (2.4) | 287 (97.6) | 1 |
| Papanicolau smear | ||||
| Abnormal | 71 (8.9) | 7 (9.9) | 64 (90.1) | 5.2 (2.05–13.27) |
| Normal | 731 (91.1) | 15 (2.1) | 716 (97.9) | 1 |
| Viral load | ||||
| Detectable | 212 (26.4) | 10 (4.7) | 202 (95.3) | 2.3 (1.01–5.60) |
| Undetectable | 590 (73.6) | 12 (2.0) | 578 (98.0) | 1 |
The factors associated with a positive CT/GC test in the multivariate logistic regression analysis were abnormal Papanicolau smear (OR 4.1; 95% CI: 1.54–11.09) and the presence of abnormal cervical discharge (OR=2.6; 95% CI: 1.02–6.71) (Table 3).
Multivariate analysis of factors associated with CT/GC positivity among women living with HIV in Brazil, 2015.
| Variables | OR | (95% CI) | p-value |
|---|---|---|---|
| Age in years (Up to 24 vs. ≥25) | 2.6 | (0.71–9.63) | 0.148 |
| Anal sex (Yes vs. No) | 1.5 | (0.62–3.70) | 0.361 |
| Papanicolau smear (Abnormal vs. normal) | 4.1 | (1.54–11.09) | 0.005 |
| Pelvic pain (Yes vs. No) | 1.3 | (0.28–2.24) | 0.656 |
| Cervical discharge (Yes vs. No) | 2.6 | (1.02–6.71) | 0.046 |
| Genital Itching (Yes vs. No) | 1.1 | (0.40–3.20) | 0.820 |
| Genital Lymphadenopathy (Yes vs. No) | 1.5 | (0.27–7.94) | 0.662 |
| Viral load (Detectable vs. Undetectable) | 1.7 | (0.67–4.06) | 0.274 |
Hosmer and Lemeshow test: X2=6.367, df: 7, p=0.498.
The variables in bold were statistically significant. They presented a p value <0.05.
Table 4 shows subanalyses performed in 377 women who reported previous STI. A total of 245 (65.0%) reported using condom more frequently after receiving the diagnosis of STI. Sixty-two (16.4%) discovered the STI after the partner told he was infected by an STI; 157 (41.6%) had STI symptoms and looked for care, and 158 (41.9%) discovered the STI in a routine consultation for another reason.
Health behavior of women living with HIV in Brazil after receiving an STI diagnosis, by condom use, 2015 (N=377).
| Variables | Condom use | No condom use | p-value |
|---|---|---|---|
| N (%) | N (%) | ||
| How did you discover the STI | 0.043 | ||
| Partner told you he was infected by an STI | 38 (61.3) | 24 (38.7) | |
| You had STI symptoms and looked for care | 116 (73.9) | 41 (26.1) | |
| You discovered it in a routine consultation for another reason | 123 (77.8) | 35 (22.2) | |
| When you were diagnosed with an STI, where did you treat it? | 0.713 | ||
| I did not treat | 5 (62.5) | 3 (37.5) | |
| Primary Health Unit | 165 (72.1) | 64 (27.9) | |
| Pharmacy | 7 (77.8) | 2 (22.2) | |
| STI clinic | 100 (73.5) | 31 (23.7) | |
| Did you tell your partner about the STI diagnosis? | 0.155 | ||
| Yes | 201 (75.6) | 65 (24.4) | |
| No | 76 (68.5) | 35 (31.5) | |
| After receiving the diagnosis did you use condoms more often? | 0.001 | ||
| Yes | 207 (84.5) | 38 (15.5) | |
| No | 70 (53.0) | 62 (47.0) | |
| After receiving the diagnosis you did you decrease your sexual activity? | 0.331 | ||
| Yes | 132 (75.9) | 42 (24.1) | |
| No | 145 (71.4) | 58 (28.6) | |
The prevalence of CT and GC in HIV-infected women in Brazil was lower than the rates reported among adolescents or young pregnant women.10,11 These results are in agreement with previous studies conducted in Rio de Janeiro6 and Manaus7 and a little higher compared to HIV-infected women from Zambia.8 The observed lower rates could be attributed to the older age of these women compared to pregnant women or adolescents.2,10,11 Due to their HIV status, it is possible that these participants were more likely to be receiving ongoing medical care and antibiotics prescriptions. These results can suggest that engaging in HIV care may play a role for controlling STI in this population.
The factors associated with positive CT/GC test in the final multivariate model were abnormal Papanicolau smear and presence of abnormal cervical discharge, which does not include inflammatory results, according with the Bethesda system.12 Some studies have linked the presence of Chlamydia in women with HPV infection and abnormal Pap smears in the general population.13 In 2011 Lehtinen et al. published a cohort study showing that women with CT at baseline were 1.78 times more likely to develop cervical intraepithelial neoplasia grade 2 due any HPV type, than those without CT.14 Screening for cervical asymptomatic CT and GC can identify women who need follow-up for HPV infection and more careful investigation of precursor lesions of cervical cancer. The association between vaginal discharge and CT and GC infection, described in our study, was not commonly reported in previous studies.15,16 The syndromic management of genital infections has not been considered effective, with this symptom being a poor predictor of cervicitis by CT and GC. Therefore, screening of asymptomatic women remains the best suited recommendation for this target population.15
Women who have reported a previous STI diagnosis were questioned about how they found out the STI. Almost half of them received treatment because they had STI symptoms and looked for care or discovered the STI in a routine consultation for another reason. The control of STI represents a unique opportunity to improve reproductive health of women living with HIV.5 Both ulcerative and non-ulcerative STI increase the risk of HIV transmission by three to 10 times, depending on the type and etiology of the STI.5 HIV-infected individuals affected by an STI have increased HIV viral load in genital secretions,4,17 thereby increasing considerably their potential of infectiousness and transmission.
The risk of HIV sexual transmission is different according to the sexual relationship. Female-to-male transmission have a risk of 0.04–0.38% per sexual act, increasing to 5.3% in case of previous history or presence of STI and genital ulcer.18 Sexual behavior has been modified worldwide, with more new sexual practices, low use of condom, and low concern about the risk of STI transmission.19 In our study, we identified that only 41.6% of the women had the diagnosis of STI because they looked for care due to presence of related symptoms. The knowledge of this diagnosis led to increased use of condoms during sexual relations. The access to STI diagnosis brings effective prevention and can change sexual behavior.
Although a cross-sectional study is not ideal for determining risk factors, its application is justified. Knowing CT and GC prevalence rates and its associated factors in HIV-infected women is important to demonstrate their susceptibility to complications caused by these infections. Given the low prevalence of some risk factors in this sample, it is possible that the number of studied women was not sufficient to find statistical association between some independent variables and CT/GC positivity. The possibility of biased answers cannot be ruled out because of the general tendency to give socially acceptable replies in face-to-face interviews.
Despite the limitations, this study suggests that screening programs must be cost-effective and must be made acceptable to patients by using non-invasive procedures. It could also be considered a preventive measure aimed to determine risk factors, or detect and treat abnormal signal and symptoms that could later cause complications. After the “Treat as Prevention” strategy, adopted in Brazil since 2013,20 people used condom less frequently, because assumed HIV could not be transmitted in the presence of viral suppression.21 At the same time, worldwide, bacterial STI, such as syphilis, CT, and NG are a high burden, mainly in people living with HIV.22 Controlling STI and identifying factors associated with such diseases continues to be an important element in the design of interventions targeting STI and as a result, HIV prevention in Brazil.
Financial supportTechnical cooperation agreement – Brazilian Department of STI, AIDS and viral hepatitis, Ministry of Health and United Nations office for drugs and crime. Project BRA/K57, process #01/2013.
Conflicts of interestThe authors declare no conflicts of interest.