To estimate the effect of tocilizumab or glucocorticoids in preventing death and intubation in patients hospitalized with SARS-CoV-2 pneumonia.
MethodsThis was a retrospective cohort study enrolling all consecutive patients hospitalized at Reggio Emilia AUSL between February the 11th and April 14th 2020 for severe COVID-19 and treated with tocilizumab or glucocorticoids (at least 80 mg/day of methylprednisolone or equivalent for at least 3 days).
The primary outcome was death within 30 days from the start of the considered therapies. The secondary outcome was a composite outcome of death and/or intubation. All patients have been followed-up until May 19th 2020, with a follow-up of at least 30 days for every patient.
To reduce confounding due to potential non-comparability of the two groups, those receiving tocilizumab and those receiving glucocorticoids, a propensity score was calculated as the inverse probability weighting of receiving treatment conditional on the baseline covariates.
Results and conclusionTherapy with tocilizumab alone was associated with a reduction of deaths (OR 0.49, 95% CI 0.21-1.17) and of the composite outcome death/intubation (OR 0.35, 95% CI 0.13-0.90) compared to glucocorticoids alone. Nevertheless, this result should be cautiously interpreted due to a potential prescription bias.
With the emergence of new viral variants1 and the vaccination programs still in the early phases in most countries, pharmacological therapy for coronavirus disease-2019 (COVID-19) is a major clinical need.
Glucocorticoids and tocilizumab have been among the few therapies that have proven a survival benefit in large randomized clinical trials (RCTs).2,3 Their effect seems to be synergic as the benefit for patients treated with tocilizumab was evident only in the glucocorticoids-treated group in the RECOVERY trial.4 Consistently, in tocilizumab RCTs, where glucocorticoids were used in a low percentage of patients or not used, tocilizumab was not able to reduce mortality rates compared with usual care or placebo.5–7 Adding tocilizumab to glucocorticoids therapy has proven effective in the treatment of giant cell arteritis8–10 and it is a treatment of choice for that vasculitis.
We can hypothesize that the combination of these two immunomodulatory therapies work on different pathways of the SARS-CoV-2-induced hyperinflammatory reaction. Interleukin-6 (IL-6) is a key inflammatory cytokine that is markedly increased in most cases of severe COVID-19 and is linked to an unfavourable outcome.11 Nevertheless, in this disease the immune dysregulation and the hyperinflammation seem to be much broader and to involve multiple cytokines and inflammatory pathways.12,13 Thus, dexamethasone might supply a wide-ranging immunomodulation providing a “back-bone” for the more selective anti-IL-6 action of tocilizumab.
However, most of the COVID-19 pathogenesis remains unknown. Although cytokine concentrations are elevated in patients with severe and critical COVID-19, the degree of cytokinemia, including IL-6 serum levels, is markedly less than that seen in patients with acute respiratory distress syndrome (ARDS) unrelated to COVID-19, sepsis, and chimeric antigen receptor (CAR) T cell-induced cytokine release syndrome. Therefore, some authors have questioned the role of a cytokine storm in COVID-19-induced organ dysfunction, and have suggested that the overall effect of SARS-CoV-2 infection is actually a hypo-immune reaction with subsequent (directly) virus-mediated tissue damage and dysregulated inflammation and that the benefit due to glucocorticoids was not related to IL-6 suppression but mainly to the other effects of GCs, including the anti-fibrotic.12 Notably, a recent study on Middle-East Respiratory Syndrome showed improved survival in patients treated with interferon-beta-1b and lopinavir/ritonavir, supporting a possible role for immunity enhancers in beta-coronaviridae-related diseases.14 Thus, further research on COVID-19 immunopathogenesis and immunotherapies is much needed.
The objective of this study was to estimate the effect of tocilizumab or glucocorticoids in preventing death and intubation in patients hospitalized with SARS-CoV-2 pneumonia.
Patients and methodsPatients and case definitionThis was a retrospective, monocentric observational cohort study performed at Reggio Emilia AUSL, at two different sites, the central research hospital of Reggio Emilia (Arcispedale Santa Maria Nuova) and Guastalla Hospital (Reggio Emilia province, Italy). All consecutive patients hospitalized between February the 11th and April 14th 2020 for severe COVID-19 pneumonia and treated with tocilizumab or glucocorticoids (at least 80 mg/die of methylprednisolone or equivalent for at least 3 days) were included.
The study was approved by Comitato Etico Area Vasta Emilia Nord.
SARS-CoV-2 infection was diagnosed at Hospital admission by a positive reverse-transcriptase polymerase chain-reaction (RT-PCR) in a respiratory tract specimen. COVID-19 pneumonia was confirmed if chest X-rays and/or high-resolution computed tomography (HRCT) scan showed suggestive findings.15–17
TreatmentTocilizumab was administered by intravenous (IV) or subcutaneous (SC) formulations. SC tocilizumab was used in some patients because IV tocilizumab was not available for a period of time.
IV tocilizumab was prescribed as 8 mg/kg (maximum dose per single infusion: 800 mg), first dose at time 0 and a second dose after 12 hours. SC tocilizumab was administered as two to four 162 mg vials simultaneously, depending on patient's weight.
Suggested clinical features for tocilizumab-therapy eligibility were evidence of a severe pneumonia (oxygen saturation at rest on room air ≤93% and/or arterial oxygen partial pressure (PaO2)/oxygen concentration (FiO2) ≤300 mmHg), the presence of exaggerated inflammatory response (body temperature> 38°C; serum C reactive protein (CRP) greater than or equal to 10 mg/dl or at least double the basal value) and absence of contraindications to tocilizumab therapy. We used CRP as its levels are a consequence of IL-6 increments, are more comparable between patients, and more promptly available at every hospital site.
Glucocorticoids therapy was administered as IV methylprednisolone 40 mg two times a day as per internal hospital protocol. As we included the first two months of Italian epidemic, glucocorticoids were still contraindicated by World Health Organization (WHO). Thus, they were mainly used as rescue therapy for patients who did not improve 3-5 days after hospitalization or when tocilizumab was less available. The suggested clinical features for methylprednisolone therapy were substantially the same as for tocilizumab: evidence of a severe pneumonia, presence of exaggerated inflammatory response (body temperature> 38°C; serum CRP greater than or equal to 10 mg/dl or at least double the basal value), absence of contraindications to glucocorticoids therapy and at least siix days from symptoms onset.
At the time tocilizumab was widely available it was given to the vast majority of patients with severe COVID-19, whereas glucocorticoids were used when tocilizumab was not available. During the days when only few doses of tocilizumab were available it was prescribed with individual patient evaluations.
To the scope of this study, we classified patients according to the first therapy received, glucocorticoids or tocilizumab. Patients who changed therapy or added the other therapy more than 24 hours after the start of first treatment were considered as receiving the first therapy, with an intention to treat approach. Patients who started both therapies on the same day (less than 24 hours) were considered in a separate group.
Data collectionData were collected from both paper and electronic clinical records. A standardized protocol with predefined laboratory tests at admission was followed for all hospitalized COVID-19 patients from March 31st. Moreover, from both paper and electronic clinical records we collected information about hospital discharge, the condition of the patients at hospital discharge, the type of respiratory support and death. Patients’ past medical history, including comorbidities and medications at home and during the hospital stay were also recorded.
Radiological dataCT scans performed at emergency room presentation were retrospectively reviewed by three radiologists in consensus, collecting the presence/absence of ground-glass opacities and consolidations, and the extension of pulmonary lesions using a visual scoring system (< 20%, 20-40%, 40-60%, and > 60% of parenchymal involvement).
Outcome measuresThe primary outcome was the occurrence of death within 30 days from diagnosis. The secondary outcome was a composite outcome of death and/or intubation. All patients have been followed-up after diagnosis up to May the 19th, 2020, with a follow-up of at least 30 days for every patient.
Occurrence of death during the follow-up was the main outcome. A secondary composite outcome of worsening during TCZ and/or glucocorticoids therapy included the patients who died or were intubated during the follow-up; patients already intubated at the moment of TCZ and/or glucocorticoids administration were not included for analyses regarding this outcome.
Statistical analysisDescriptive statistics for continuous variables were reported as median and inter-quartile range (IQR).
To avoid the loss of the sample size and to reduce bias estimates in the logistic model, we used a multiple imputation truncated regression to fill in missing values of the continuous variables.18
To reduce confounding due to non-comparability of the two groups, those receiving tocilizumab and those receiving glucocorticoids, a propensity score was calculated as the inverse probability weighting of receiving treatment conditional on the baseline covariates reported in supplementary Table 1. These probabilities were obtained by fitting a logistic regression model of treatment status on whatever characteristics of each subject. Furthermore, multivariate analysis was performed using a logistic regression model to measure the odds ratio, with relative 95% CI, of death for COVID-19 and of intubation or death, adjusting for pre-existing conditions, disease related conditions and propensity score.
All main analyses were conducted excluding patients who received both treatments on the same day. As sensitivity analyses, we included patients who received both treatments as third separate group. Two different propensity scores were computed for this analysis, one considering patients with both treatments as receiving tocilizumab, and one as receiving glucocorticoids. The two scores were then included alternatively in logistic models to see the robustness of the results.
All the analyses were conducted with STATA v.13.
ResultsA total of 295 patients were included in the study, 135 in the tocilizumab group (75 intravenous, 60 subcutaneous), 142 in the glucocorticoids group and 18 in the combination group (3 intravenous tocilizumab, 15 subcutaneous). The only glucocorticoid used was methylprednisolone. Demographic, clinical, serological and radiological features of patients are summarized in Supplementary Table 1.
The full dose (80 mg/day) of methylprednisolone was administered for a mean of 5.45 days (range 3-17 days) with a slight difference between the methylprednisolone-alone group (mean 5.47 days, range 3-17 days) and methylprednisolone-tocilizumab combination group (mean 5.28 days, range 3-8 days).
Patients who received tocilizumab compared to those who received methylprednisolone were younger, (median age 65 vs. 73 years), had less comorbidities, in particular COPD, dementia, chronic kidney disease, heart failure, arrhythmia; they were more frequently obese even if they had lower prevalence of dyslipidaemia. Regarding previous use of drugs, tocilizumab patients had received more frequently angiotensin II receptor blocker and less frequently ACE-inhibitors. Furthermore, they had been less frequently treated with methylprednisolone before hospital admission. Time from symptoms onset to treatment was similar in the two groups, but symptoms were slightly different: those receiving tocilizumab had more frequently fever and cough, less myalgia and asthenia, but similar O2 saturation; greater extent of lung parenchyma involvement, as well CRP and IL-6 were higher while a smaller proportion had high level of troponin and neutrophils. Time from hospitalization to treatment was also similar in the two groups (tocilizumab: median 2 days – IQR 1-4 days; methylprednisolone: median 2 days – IQR 1-6 days).
From March 11 to March 23 tocilizumab was available and administered to the vast majority of patients, then the availability of drug decreased and the proportion of patients treated with methylprednisolone increased (Supplementary Figure 1).
Fifteen patients underwent orotracheal intubation in both tocilizumab and methylprednisolone groups, while one was intubated in the combination group. Nineteen patients died in the tocilizumab group, 38 in the methylprednisolone group and three in the combination group. Out of 135 patients in the tocilizumab-alone five underwent orotracheal intubation and died, compared to 7 out of 142 in the methylprednisolone group, and 1 out of 18 in the tocilizumab plus methylprednisolone group. The median follow-up was similar in the three groups (14 days for both tocilizumab and methylprednisolone groups, 13 for the combined group) (Supplementary Table 1).
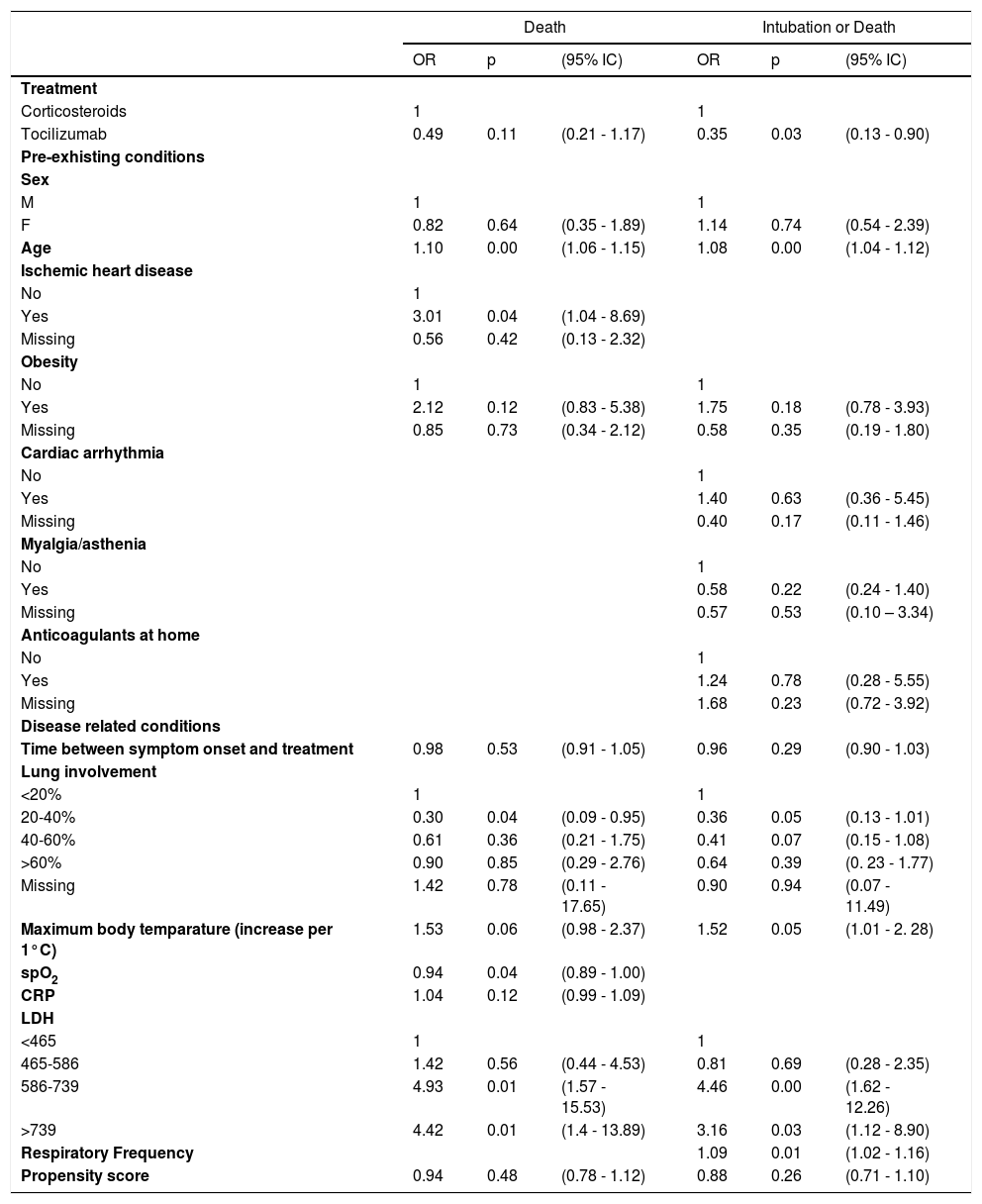
Table 1 shows multivariate logistic regression related to clinical outcomes (death and composite outcome death/intubation). Therapy with tocilizumab alone was associated with a reduction of deaths (OR 0.49, 95% CI 0.21-1.17) and of the composite outcome death/intubation (OR 0.35, 95% CI 0.13-0.90) compared to methylprednisolone alone. Propensity scores were not influential in the model, while both outcomes were associated with age, obesity, fever, lung involvement (as protective), and LDH, while ischaemic heart disease and saturation were associated only with death and respiratory frequency with intubation or death. Among these variables, only obesity, fever, and lung involvement were confounders, because other variables are not associated with treatment.
Multivariate logistic regression related to clinical outcomes.
Model are also adjusted for calendar time.
The associations between treatment and outcomes were robust to different sensitivity analyses: including also patients treated with both drugs the difference remained, while patients treated with both drugs had higher risk of death and death or intubation, even if the difference could be due to chance. Different ways to construct the propensity score did not change results.
DiscussionOur study suggests that patients treated with tocilizumab had a lower likelihood of the composite outcome death or intubation compared to patients treated with methylprednisolone only. Even though this result is consistent with previous literature19,20 and was confirmed in multivariate analysis, it should be carefully interpreted, as the two groups differ in key demographic and clinical characteristics. Notably, patients in the tocilizumab group were younger compared to the methylprednisolone group. This may reflect a prescription bias as tocilizumab was preferred in younger patients with less comorbidities. In fact, the availability of tocilizumab was not constant during the study period, allowing the treatment of the vast majority of patients in the first two weeks of the study period, then a shortage occurred and clinicians were forced to select more carefully patients that could have a benefit from the therapy. This selection introduced a prescription bias that we tried to account for with a strategy of double adjusting, with propensity score and including the most influential variables in the models. The observed associations were robust to sensitivity analyses, but residual confounding could not be excluded in the presence of prescription bias.
No improvement in survival rate was observed in the tocilizumab plus methylprednisolone combined therapy. However, this analysis was limited by the small number of patients evaluated (only 18), whereas the size of the other two groups was much larger and similar. Furthermore, even if we considered in this group only patient who started the two treatments on the same day (<24h) we cannot exclude that some of these patients received the second treatment as a rescue therapy because the patient did not respond to the first one. Thus, the reduced survival in the combination group is likely to suffer from a selection bias as the tocilizumab-methylprednisolone combination was often used as a rescue therapy in the most severe patients.
Our study has limitations, mostly related to its retrospective and observational nature. There is an evident selection bias as the two main groups (tocilizumab alone and methylprednisolone alone) have different baseline characteristics, most notably almost eight years difference in the median age. This reflects the use of methylprednisolone in the first months of the epidemic at our centres, as they were used as compassionate therapy in most severe patients because WHO had not recommended their use at the time.
The cohort included in this study is highly selected accordingly to the criteria for prescribing the two drugs in that period. This selection introduces a selection bias that should not necessarily affect the comparison between the two drugs, but makes not interpretable the association between other covariates and the outcomes, as it is evident for the protective effect of lung involvement, which is a strong negative prognostic factors in unselected cohort where the present study was nested.15
Few patients were treated with a combination therapy of tocilizumab and methylprednisolone and they were a highly selected group, in which probably signs of bad prognosis of treatment failure were already appreciable by the clinicians when the second therapy was administered. Thus our data provide no information from real life practice for the combined therapy that recent RCTs suggest to be the most effective.4,21 It must be noticed that in these studies2,21 a clear benefit from anti-IL-6 agents treatment was witnessed only in patients receiving associated glucocorticoids. These data were confirmed in a recent WHO rapid meta-analysis22 were no clear improvement in 28-day mortality rate was not significantly improved in patients not receiving glucocorticoids.
Some strengths should be acknowledged as well. The two main groups of patients have analogous size and the patients were homogeneously followed-up using a common standardized protocol at the two hospital sites included. In addition, from March 31st, standardized blood tests and CT scan protocol was applied to all patients admitted with COVID-19. Nevertheless, there is evidence of prescription bias, acting particularly when shortage of tocilizumab occurred. We tried to account for the lack of comparability of the two groups using a propensity score that allowed to include a large number of potential confounders. Furthermore, we included in the final multivariate model all the variables that were still associated with outcomes. This analysis minimizes the risk of residual confounding, even if it cannot eliminate it.
FundingNo dedicated funding was used to conduct this study.
The following are members of the Reggio Emilia COVID-19 Working Group:
Massimo Costantini, Giulio Formoso, Emanuela Bedeschi, Cinzia Perilli, Elisabetta Larosa, Eufemia Bisaccia, Ivano Venturi, Cinzia Campari, Francesco Gioia, Serena Broccoli, Marta Ottone, Pierpaolo Pattacini, Giovanni Dolci, Romina Corsini, Giulia Besutti, Matteo Revelli, Valentina Iotti, Lucia Spaggiari, Pamela Mancuso, Paolo Giorgi-Rossi, Andrea Nitrosi, Marco Foracchia, Rossana Colla, Alessandro Zerbini, Marco Massari, Anna Maria Ferrari, Mirco Pinotti, Nicola Facciolongo, Ivana Lattuada, Laura Trabucco, Stefano De Pietri, Giorgio Francesco Danelli, Laura Albertazzi, Enrica Bellesia, Simone Canovi, Mattia Corradini, Tommaso Fasano, Elena Magnani, Annalisa Pilia, Alessandra Polese, Silvia Storchi Incerti, Piera Zaldini, Efrem Bonelli, Bonanno Orsola, Elisabetta Teopompi, Carlo Salvarani.