Understanding the intricate dynamics between different waves of the COVID-19 pandemic and the corresponding variations in clinical outcomes is essential for informed public health decision-making. Comprehensive insights into these fluctuations can guide resource allocation, healthcare policies, and the development of effective interventions. This study aimed to compare the characteristics and clinical outcomes of COVID-19 at peak transmission points by including all patients attended during the first four pandemic waves in a referral center in Colombia.
Material and methodsIn a prospective observational study of 2733 patients, clinical and demographic data were extracted from the Fundacion Valle de Lili's COVID-19 Registry, focusing on ICU admission, Invasive Mechanical Ventilation (IMV), length of hospital stay, and mortality.
ResultsOur analysis unveiled substantial shifts in patient care patterns. Notably, the proportion of patients receiving glucocorticoid therapy and experiencing secondary infections exhibited a pronounced decrease across waves (p < 0.001). Remarkably, there was a significant reduction in ICU admissions (62.83% vs. 51.23% vs. 58.23% vs. 46.70 %, p < 0.001), Invasive Mechanical Ventilation (IMV) usage (39.25% vs. 32.22% vs. 31.22% vs. 21.55 %, p < 0.001), and Length of Hospital Stay (LOS) (9 vs. 8 vs. 8 vs. 8 days, p < 0.001) over the successive waves. Surprisingly, hospital mortality remained stable at approximately 18‒20 % (p > 0.05). Notably, vaccination coverage with one or more doses surged from 0 % during the initial waves to 66.71 % in the fourth wave.
ConclusionsOur findings emphasize the critical importance of adapting healthcare strategies to the evolving dynamics of the pandemic. The reduction in ICU admissions, IMV utilization, and LOS, coupled with the rise in vaccination rates, underscores the adaptability of healthcare systems. Hospital mortality's persistence may warrant further exploration of treatment strategies. These insights can inform public health responses, helping policymakers allocate resources effectively and tailor interventions to specific phases of the pandemic.
Coronavirus Disease 2019 (COVID-19) has caused a global health crisis with a cumulative incidence of more than 622.5 million cases and 6.6 million deaths reported by October 2022. Latin American countries have been among the most affected, with Colombia ranking fourth in confirmed cases after Brazil, Argentina and Mexico, with 6.3 million confirmed cases and 141,000 deaths.1
Differences in clinical outcomes of patients between waves of the COVID-19 pandemic have been reported previously.2,3 The emerged omicron variant has been shown to have high transmissibility but not high virulence, resulting in lower hospitalization and mortality rates in population-based studies.4
To date, there is no widely accepted definition of a COVID-19 pandemic wave.5 Yet, a distinguishing feature that should be preserved throughout time is a rising or declining number of cases. Although the waves do not occur at the same time in all countries, the following waves are more severe and result in more deaths, especially in nations where immunization is not widely available.6
The COVID-19 immunization campaign in Colombia started in February 2021 and was carried out in stages, giving front-line healthcare providers and the elderly priority.7,8 All age groups began receiving vaccinations in July 2021, and by February 2022, more than 60 % of the Cali and the Colombian population had received the recommended vaccination scheme (two doses of any vaccine or one Ad26.COV2.S).7,8 Despite this, there is limited data on the clinical traits and prognoses of COVID-19 patients admitted to hospitals in Colombia during the several pandemic waves, both before and after immunization. Assessment of these traits is essential to determine if public health initiatives like immunization and modifications to clinical treatment practices were effective.9
This study aim is to compare the experiences and clinical outcomes of COVID-19 patients who were admitted in a Latin American university hospital in Cali, Colombia, during the first four waves of the pandemic. The results of this study will enable researchers, policy makers and health professionals to better understand the effects of the pandemic and to use this knowledge to inform future public health initiatives.
Materials and methodsPatients and settingA single-center observational prospective analysis was conducted at Fundación Valle del Lili (FVL), a non-profit university hospital located in Cali, a major city from Colombia, with a population of 2.5 million inhabitants. Patients aged ≥ 18 years-old with confirmed SARS-CoV-2 infection by viral real-time RT-PCR test Assay, viral antigen detection, or presence of SARS-CoV2 antibodies with high clinical suspicion, were included from the first four waves of the pandemic, defined as one month before and after the highest peaks of the number of cases in the pandemic for Colombia. The first wave from June to August 2020, the second from November 2020 to January 2021, the third from May to July 2021 and the fourth from December 2021 to February 2022. Patients with previous diagnosis and resolved SARS-CoV-2 infection were excluded.
SARS-CoV-2 diagnostic testsSARS-CoV-2 infections were diagnosed with nasopharyngeal swabs using the CDC 2019-nCoV Real-Time RT-PCR Diagnostic Panel protocol (CDC, USA), VIASURE® SARS-CoV-2 Real-Time PCR Detection Kit (Certest Biotec S.L.), Allplex™ 2019-nCoV Assay (Seegene Inc), or AccuPower® SARS-CoV-2 Multiplex Real-Time RT-PCR Kit (Bioneer Corporation). In other patients, it was used the BD Veritor™ SARS-CoV-2 antigen test (Becton), SARS-CoV-2 Rapid Antigen Test (Roche Diagnostics). Or IgG/IgM antibodies assay (Abbott Rapid Diagnostics). The choice of the diagnostic test was defined by the treating physician according to the clinical case and the availability of the test.
Source of informationDemographic and clinical data were obtained from the COVID-19 Registry (RECOVID-19) at FVL. This registry was an institutional initiative that was developed at the beginning of the pandemic in which the clinical, paraclinical and microbiological data of all patients with confirmed cases of COVID-19 treated at FVLsince the beginning of the pandemic.10
Demographic and clinical data were sourced from the COVID-19 Registry (RECOVID-19), an initiative launched at the pandemic's outset to comprehensively gather data from every patient treated for confirmed COVID-19 at FVL. Information was registered on a web-based registry using Research Electronic Data Capture (REDCap), a secure software platform designed for research study data capture. The clinical data entry staff carried out daily systematic data collection, with sociodemographic and clinical information being directly extracted from the patient's electronic health records managed by the mySiss® SAP system, alongside diagnostic tests and laboratory results from the enterprise platform. This active, combined retrospective and prospective, search for cases ensured the registry's final version was organized into three main data collection forms: admission, follow-ups, and outcomes. It enabled systematic tracking of all hospitalized patients from admission through days 1, 2, 3, 4, 5, 7, 14, and 28, capturing everything from sociodemographic and epidemiological details to medical history, physical examination findings, laboratory and imaging results, severity assessments, treatments administered, and complications encountered. Such a comprehensive approach supported detailed outcome analyses, including the duration of hospitalization and discharge status.
Variables and clinical dataPatients were followed during the hospital stay up to the first 28 days after admission. The medical history included was extracted from the patient's medical history at the time of admission. The COVID-19 vaccines available in Colombia during this period was BNT162b2 (Pfizer/BioNTech), CoronaVac (Sinovac), mRNA-1273 (Moderna), AZD1222, ChAdOx1 (Oxford-AstraZeneca) or Ad26.COV2.S (Janssen). COVID-19 vaccine. Vaccination was classified as heterologous when the participants received two different kinds of vaccine or homologous when all vaccine doses were of the same one.
The primary outcomes included intensive care unit admission, invasive mechanical ventilation requirement, hospital length of stay, and hospital mortality. Secondary outcomes included the use of glucocorticoids and secondary infections defined by the presence of clinical manifestations compatible with infection and the isolation of an etiologic agent in cultures (sputum, endotracheal aspirate, bronchoalveolar lavage), blood samples, urine or molecular detection tests and/or antigen detection methods. The waves were demarcated as the period of three months in which a sustained rise over time was observed. The time-lapse of the wave was defined as one month before and one month after the highest peaks of cases.
Sample sizeSince the objective of this study was to compare the characteristics and clinical outcomes of COVID-19 at peak transmission points, all patients attended during the four waves of highest case transmission were included. Patients between waves were excluded. A minimal sample size of 317 patients per wave was calculated to find differences in proportions of 3 % or more between the four pandemic periods. The expected power was 80 %, an alpha of 0.05 and an effect size between 0.15 and 0.18. For the estimation of the sample, a population of 2 million people was assumed (approximate population of Cali, Colombia). The effect size was estimated using the following equation:
Where:w is the effect size calculated using the Chi-Squared test.
χ² is the Chi-Squared statistic obtained from the test.
n is the total sample size.
The calculations were performed in R using the pwr.chisq.test function of the pwr library.
Statistical analysisA descriptive analysis was performed, categorical variables were presented as frequencies and proportions, and continuous variables were presented as median with Interquartile Range (IQR) or mean with standard deviation, considering the variable distribution. A subgroup analysis based on age groups or decades was performed for the outcomes: invasive mechanical ventilation requirement, intensive care unit admission, Length of Hospital Stay (LOS), and in-hospital mortality. Chi-Square tests were used on categorical variables comparisons, Kruskal-Wallis tests were used to assess non-normally distributed data sets; p-values of < 0.050 were considered statistically significant. Statistical analysis was performed using Stata 17 (StataCorp, College Station, TX, USA) and R Statistical Software (v4.1.2; R Core Team 2021).
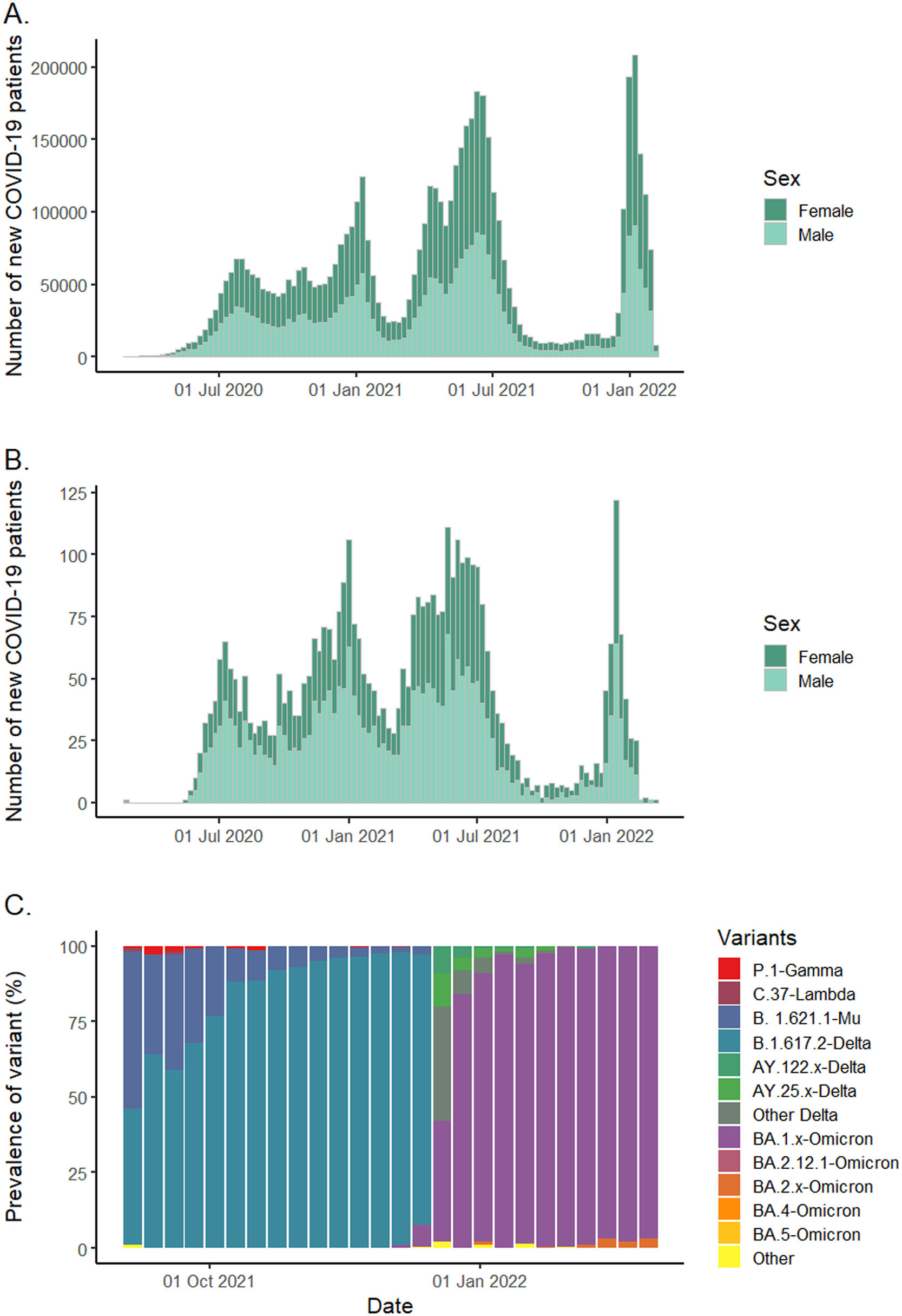
ResultsFrom June 2020 to February 2022, 6,039,022 COVID-19 cases were reported in Colombia of which 4275 patients were seen at FVL and 2733 were included (530 patients consulted in the first wave, 568 in the second wave, 1166 in the third wave and 469 in the fourth wave). The patterns of COVID-19 waves were very similar in our hospital compared to national patterns (Fig. 1, Panel A and B).
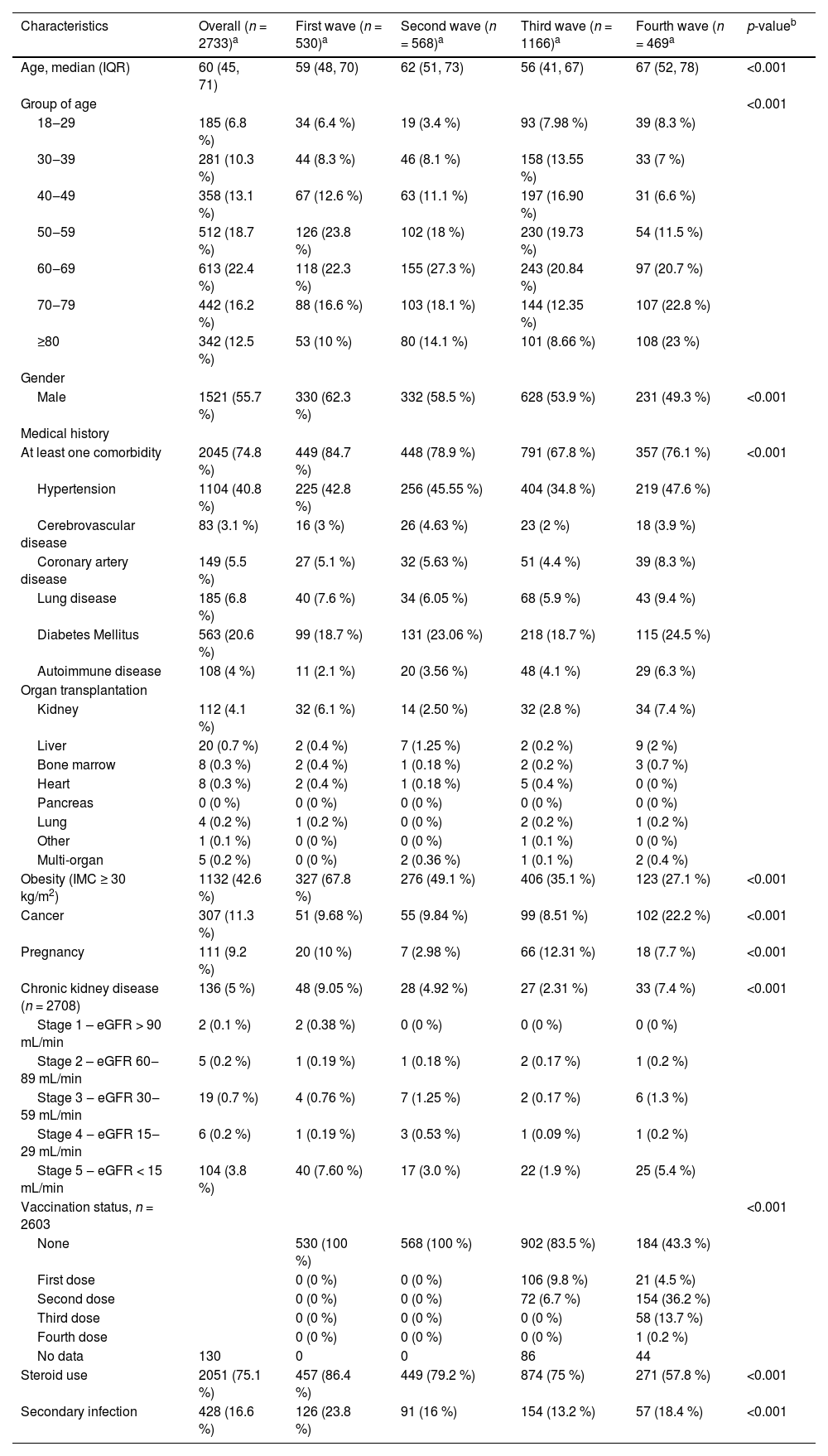
Slightly over the half of the patients were male (55.6 %), with a mean age of 60 years, and 74.8 % had at least one comorbidity. Table 1 summarizes the characteristics of the hospitalized patients with SARS-CoV-2 infection stratified by wave. The second and third wave included a higher proportion of patients older than 50 years old. For the third wave, cases under 50-years-old were 38.4 % compared to the other waves which was less than 30 %. A decreasing proportion of males through waves were recorded. Hypertension (40.8 %), diabetes (20.6 %) and obesity (42.6 %) were common comorbidities during the four waves.
Baseline characteristics of hospitalized patients with SARS-CoV-2 infection.
| Characteristics | Overall (n = 2733)a | First wave (n = 530)a | Second wave (n = 568)a | Third wave (n = 1166)a | Fourth wave (n = 469a | p-valueb |
|---|---|---|---|---|---|---|
| Age, median (IQR) | 60 (45, 71) | 59 (48, 70) | 62 (51, 73) | 56 (41, 67) | 67 (52, 78) | <0.001 |
| Group of age | <0.001 | |||||
| 18‒29 | 185 (6.8 %) | 34 (6.4 %) | 19 (3.4 %) | 93 (7.98 %) | 39 (8.3 %) | |
| 30‒39 | 281 (10.3 %) | 44 (8.3 %) | 46 (8.1 %) | 158 (13.55 %) | 33 (7 %) | |
| 40‒49 | 358 (13.1 %) | 67 (12.6 %) | 63 (11.1 %) | 197 (16.90 %) | 31 (6.6 %) | |
| 50‒59 | 512 (18.7 %) | 126 (23.8 %) | 102 (18 %) | 230 (19.73 %) | 54 (11.5 %) | |
| 60‒69 | 613 (22.4 %) | 118 (22.3 %) | 155 (27.3 %) | 243 (20.84 %) | 97 (20.7 %) | |
| 70‒79 | 442 (16.2 %) | 88 (16.6 %) | 103 (18.1 %) | 144 (12.35 %) | 107 (22.8 %) | |
| ≥80 | 342 (12.5 %) | 53 (10 %) | 80 (14.1 %) | 101 (8.66 %) | 108 (23 %) | |
| Gender | ||||||
| Male | 1521 (55.7 %) | 330 (62.3 %) | 332 (58.5 %) | 628 (53.9 %) | 231 (49.3 %) | <0.001 |
| Medical history | ||||||
| At least one comorbidity | 2045 (74.8 %) | 449 (84.7 %) | 448 (78.9 %) | 791 (67.8 %) | 357 (76.1 %) | <0.001 |
| Hypertension | 1104 (40.8 %) | 225 (42.8 %) | 256 (45.55 %) | 404 (34.8 %) | 219 (47.6 %) | |
| Cerebrovascular disease | 83 (3.1 %) | 16 (3 %) | 26 (4.63 %) | 23 (2 %) | 18 (3.9 %) | |
| Coronary artery disease | 149 (5.5 %) | 27 (5.1 %) | 32 (5.63 %) | 51 (4.4 %) | 39 (8.3 %) | |
| Lung disease | 185 (6.8 %) | 40 (7.6 %) | 34 (6.05 %) | 68 (5.9 %) | 43 (9.4 %) | |
| Diabetes Mellitus | 563 (20.6 %) | 99 (18.7 %) | 131 (23.06 %) | 218 (18.7 %) | 115 (24.5 %) | |
| Autoimmune disease | 108 (4 %) | 11 (2.1 %) | 20 (3.56 %) | 48 (4.1 %) | 29 (6.3 %) | |
| Organ transplantation | ||||||
| Kidney | 112 (4.1 %) | 32 (6.1 %) | 14 (2.50 %) | 32 (2.8 %) | 34 (7.4 %) | |
| Liver | 20 (0.7 %) | 2 (0.4 %) | 7 (1.25 %) | 2 (0.2 %) | 9 (2 %) | |
| Bone marrow | 8 (0.3 %) | 2 (0.4 %) | 1 (0.18 %) | 2 (0.2 %) | 3 (0.7 %) | |
| Heart | 8 (0.3 %) | 2 (0.4 %) | 1 (0.18 %) | 5 (0.4 %) | 0 (0 %) | |
| Pancreas | 0 (0 %) | 0 (0 %) | 0 (0 %) | 0 (0 %) | 0 (0 %) | |
| Lung | 4 (0.2 %) | 1 (0.2 %) | 0 (0 %) | 2 (0.2 %) | 1 (0.2 %) | |
| Other | 1 (0.1 %) | 0 (0 %) | 0 (0 %) | 1 (0.1 %) | 0 (0 %) | |
| Multi-organ | 5 (0.2 %) | 0 (0 %) | 2 (0.36 %) | 1 (0.1 %) | 2 (0.4 %) | |
| Obesity (IMC ≥ 30 kg/m2) | 1132 (42.6 %) | 327 (67.8 %) | 276 (49.1 %) | 406 (35.1 %) | 123 (27.1 %) | <0.001 |
| Cancer | 307 (11.3 %) | 51 (9.68 %) | 55 (9.84 %) | 99 (8.51 %) | 102 (22.2 %) | <0.001 |
| Pregnancy | 111 (9.2 %) | 20 (10 %) | 7 (2.98 %) | 66 (12.31 %) | 18 (7.7 %) | <0.001 |
| Chronic kidney disease (n = 2708) | 136 (5 %) | 48 (9.05 %) | 28 (4.92 %) | 27 (2.31 %) | 33 (7.4 %) | <0.001 |
| Stage 1 – eGFR > 90 mL/min | 2 (0.1 %) | 2 (0.38 %) | 0 (0 %) | 0 (0 %) | 0 (0 %) | |
| Stage 2 – eGFR 60‒89 mL/min | 5 (0.2 %) | 1 (0.19 %) | 1 (0.18 %) | 2 (0.17 %) | 1 (0.2 %) | |
| Stage 3 ‒ eGFR 30‒59 mL/min | 19 (0.7 %) | 4 (0.76 %) | 7 (1.25 %) | 2 (0.17 %) | 6 (1.3 %) | |
| Stage 4 ‒ eGFR 15‒29 mL/min | 6 (0.2 %) | 1 (0.19 %) | 3 (0.53 %) | 1 (0.09 %) | 1 (0.2 %) | |
| Stage 5 ‒ eGFR < 15 mL/min | 104 (3.8 %) | 40 (7.60 %) | 17 (3.0 %) | 22 (1.9 %) | 25 (5.4 %) | |
| Vaccination status, n = 2603 | <0.001 | |||||
| None | 530 (100 %) | 568 (100 %) | 902 (83.5 %) | 184 (43.3 %) | ||
| First dose | 0 (0 %) | 0 (0 %) | 106 (9.8 %) | 21 (4.5 %) | ||
| Second dose | 0 (0 %) | 0 (0 %) | 72 (6.7 %) | 154 (36.2 %) | ||
| Third dose | 0 (0 %) | 0 (0 %) | 0 (0 %) | 58 (13.7 %) | ||
| Fourth dose | 0 (0 %) | 0 (0 %) | 0 (0 %) | 1 (0.2 %) | ||
| No data | 130 | 0 | 0 | 86 | 44 | |
| Steroid use | 2051 (75.1 %) | 457 (86.4 %) | 449 (79.2 %) | 874 (75 %) | 271 (57.8 %) | <0.001 |
| Secondary infection | 428 (16.6 %) | 126 (23.8 %) | 91 (16 %) | 154 (13.2 %) | 57 (18.4 %) | <0.001 |
Patients admitted in the third wave had a lower prevalence of hypertension (34.8 %, compared to the other waves that were up to 40 %) and cerebrovascular diseases (2 %, in relationship with the trend higher than 3 %). In the fourth wave the history of cancer was significantly higher (22.2 %), while the trend in the other waves didn't overtake 10 %.
The vaccination was only available until the third wave. At the end of the study period the proportion of patients that received at least one dose of vaccination against COVID-19 was 56.7 %.
In addition, when contrasting the variants circulating in Colombia with respect to the waves of cases during the study period, it became evident that the end of the third wave coincided with a high circulation of B.1.617.2-Delta and B.1.621.1-Mu, while the fourth wave coincided with a higher circulation of BA.1.x-Omicron (Fig. 1, Panel C). Genomic surveillance in Colombia started later, so no public data were available regarding circulating variants during the first two waves.
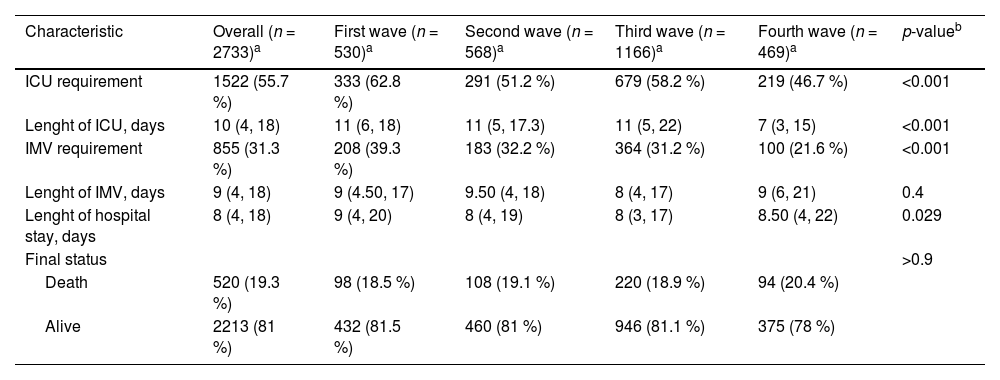
Overall, ICU admissions were more frequent during the first wave (62.83 %), reflecting the initial challenges faced in managing severe cases of COVID-19. As the pandemic progressed, there was a significant reduction in the rate of ICU admissions across the successive waves: 51.23 % in the second wave, 58.23 % in the third wave, and finally down to 46.70 % in the fourth wave (p < 0.001). Additionally, the data revealed significant differences in ICU requirements among waves. Table 2 summarizes the outcomes of hospitalized patients stratified by age, highlighting these differences.
Outcomes in hospitalized patients with SARS-CoV-2 infection.
| Characteristic | Overall (n = 2733)a | First wave (n = 530)a | Second wave (n = 568)a | Third wave (n = 1166)a | Fourth wave (n = 469)a | p-valueb |
|---|---|---|---|---|---|---|
| ICU requirement | 1522 (55.7 %) | 333 (62.8 %) | 291 (51.2 %) | 679 (58.2 %) | 219 (46.7 %) | <0.001 |
| Lenght of ICU, days | 10 (4, 18) | 11 (6, 18) | 11 (5, 17.3) | 11 (5, 22) | 7 (3, 15) | <0.001 |
| IMV requirement | 855 (31.3 %) | 208 (39.3 %) | 183 (32.2 %) | 364 (31.2 %) | 100 (21.6 %) | <0.001 |
| Lenght of IMV, days | 9 (4, 18) | 9 (4.50, 17) | 9.50 (4, 18) | 8 (4, 17) | 9 (6, 21) | 0.4 |
| Lenght of hospital stay, days | 8 (4, 18) | 9 (4, 20) | 8 (4, 19) | 8 (3, 17) | 8.50 (4, 22) | 0.029 |
| Final status | >0.9 | |||||
| Death | 520 (19.3 %) | 98 (18.5 %) | 108 (19.1 %) | 220 (18.9 %) | 94 (20.4 %) | |
| Alive | 2213 (81 %) | 432 (81.5 %) | 460 (81 %) | 946 (81.1 %) | 375 (78 %) |
In general, younger patients (aged 49 years or younger) had a higher ICU and IMV requirement in the third wave, in contrast to the older groups (aged 60-years or older) who mainly were admitted to ICU during the second wave. The trend for IMV requirement mirrored this pattern, with the younger cohort showing a higher proportion of IMV use during the third wave. Notably, the overall use of IMV was more frequent in the first wave at 39.3 %, which then decreased to 32.2 % in the second wave, 31.2 % in the third wave, and significantly dropped to 21.6 % in the fourth wave (p < 0.001). This observed decrease in IMV usage across the waves aligns with the overall trend of reduced ICU admissions.
The hospital LOS was longest during the first wave, with a median of 9 days, and remained consistent at 8 days for the second and third waves. A minor uptick to a median of 8.5 days occurred in the fourth wave. Among different age groups, patients between 60 and 79 years experienced the most extended LOS. However, during the third wave, the 50‒59 age range saw the lengthiest hospital stays, whereas there was a gradual decrease in LOS for the eldest group (80 years or older) throughout the waves. The in-hospital mortality remained stable through this wave analysis, with rates oscillating between approximately 18‒20 %. However, when stratified by age, we found that patients aged 50–59 years-old had lower mortality during the first two waves, followed by a significant increase in the third and fourth wave. The distribution of mortality according to age and waves was illustrated in Table A1.
ICU mortality was 82.3 %, with a significant difference comparing non-ICU patients (17.7 %), see Table 2.
A higher utilization of corticosteroids was noted during the first wave, with a subsequent pronounced decrease across the later waves: 86.3 % in the first wave, 79.2 % in the second, 74.9 % in the third, and 57.8 % in the fourth (p < 0.001). Similarly, the incidence of secondary infections was highest initially at 23.8 %, dropping to 16 % in the second wave, further declining to 13.2 % in the third, with a slight increase to 18.4 % in the fourth wave (p < 0.001).
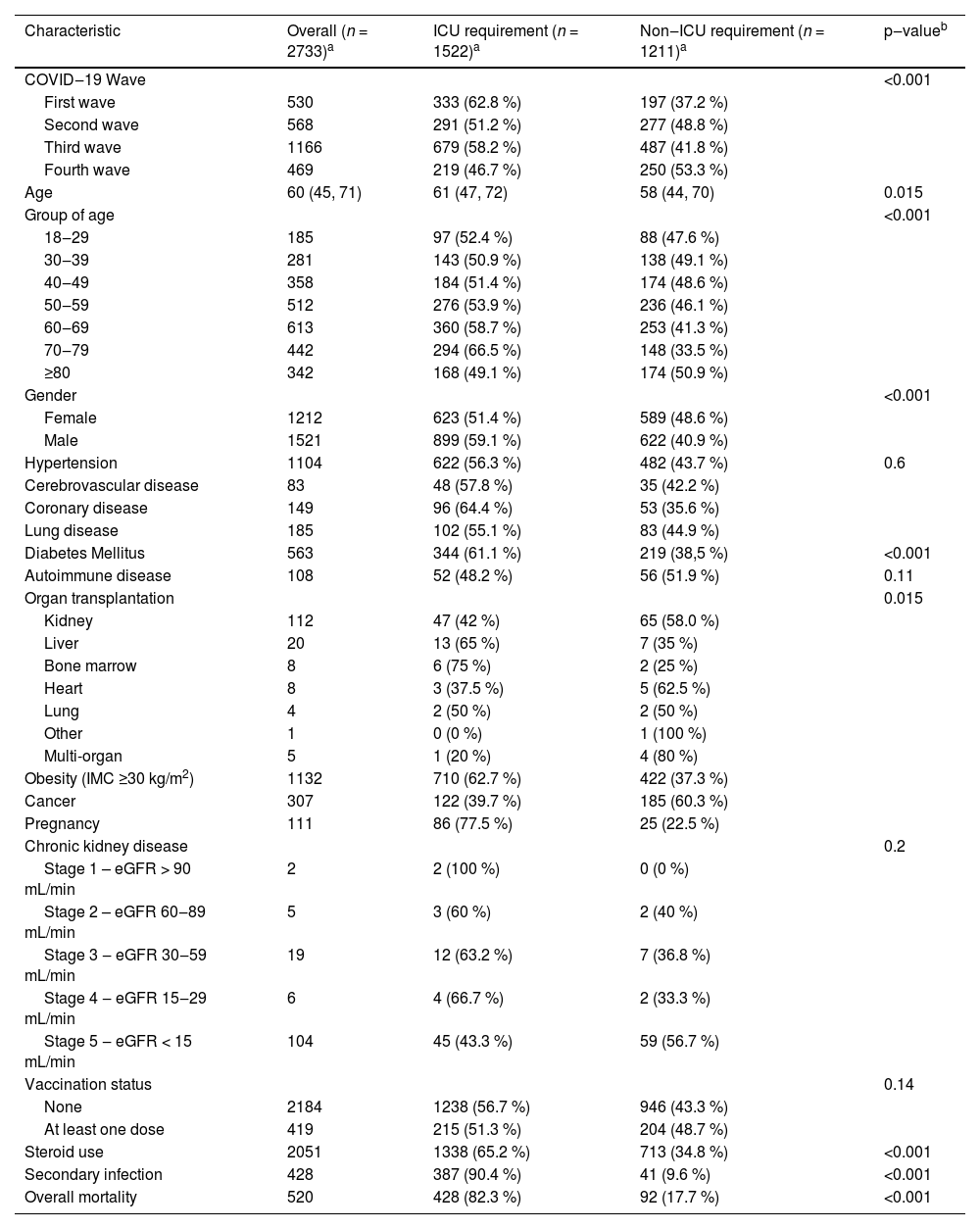
Among ICU patients (Table 3), a higher proportion of patients had a history of diabetes and obesity, and in-hospital mortality; however, chronic kidney disease, cancer, and solid organ transplantation were mainly managed at the general ward. Furthermore, there were no significant differences among non-ICU and ICU patients regarding the vaccination status; 16.7 % of ICU patients had at least one dose of vaccine against SARS-CoV-2 vs. 15.9 % admitted to the general ward.
Clinical comparison between patients with SARS-CoV-2 infection requiring ICU versus those who did not.
| Characteristic | Overall (n = 2733)a | ICU requirement (n = 1522)a | Non‒ICU requirement (n = 1211)a | p‒valueb |
|---|---|---|---|---|
| COVID‒19 Wave | <0.001 | |||
| First wave | 530 | 333 (62.8 %) | 197 (37.2 %) | |
| Second wave | 568 | 291 (51.2 %) | 277 (48.8 %) | |
| Third wave | 1166 | 679 (58.2 %) | 487 (41.8 %) | |
| Fourth wave | 469 | 219 (46.7 %) | 250 (53.3 %) | |
| Age | 60 (45, 71) | 61 (47, 72) | 58 (44, 70) | 0.015 |
| Group of age | <0.001 | |||
| 18‒29 | 185 | 97 (52.4 %) | 88 (47.6 %) | |
| 30‒39 | 281 | 143 (50.9 %) | 138 (49.1 %) | |
| 40‒49 | 358 | 184 (51.4 %) | 174 (48.6 %) | |
| 50‒59 | 512 | 276 (53.9 %) | 236 (46.1 %) | |
| 60‒69 | 613 | 360 (58.7 %) | 253 (41.3 %) | |
| 70‒79 | 442 | 294 (66.5 %) | 148 (33.5 %) | |
| ≥80 | 342 | 168 (49.1 %) | 174 (50.9 %) | |
| Gender | <0.001 | |||
| Female | 1212 | 623 (51.4 %) | 589 (48.6 %) | |
| Male | 1521 | 899 (59.1 %) | 622 (40.9 %) | |
| Hypertension | 1104 | 622 (56.3 %) | 482 (43.7 %) | 0.6 |
| Cerebrovascular disease | 83 | 48 (57.8 %) | 35 (42.2 %) | |
| Coronary disease | 149 | 96 (64.4 %) | 53 (35.6 %) | |
| Lung disease | 185 | 102 (55.1 %) | 83 (44.9 %) | |
| Diabetes Mellitus | 563 | 344 (61.1 %) | 219 (38,5 %) | <0.001 |
| Autoimmune disease | 108 | 52 (48.2 %) | 56 (51.9 %) | 0.11 |
| Organ transplantation | 0.015 | |||
| Kidney | 112 | 47 (42 %) | 65 (58.0 %) | |
| Liver | 20 | 13 (65 %) | 7 (35 %) | |
| Bone marrow | 8 | 6 (75 %) | 2 (25 %) | |
| Heart | 8 | 3 (37.5 %) | 5 (62.5 %) | |
| Lung | 4 | 2 (50 %) | 2 (50 %) | |
| Other | 1 | 0 (0 %) | 1 (100 %) | |
| Multi-organ | 5 | 1 (20 %) | 4 (80 %) | |
| Obesity (IMC ≥30 kg/m2) | 1132 | 710 (62.7 %) | 422 (37.3 %) | |
| Cancer | 307 | 122 (39.7 %) | 185 (60.3 %) | |
| Pregnancy | 111 | 86 (77.5 %) | 25 (22.5 %) | |
| Chronic kidney disease | 0.2 | |||
| Stage 1 – eGFR > 90 mL/min | 2 | 2 (100 %) | 0 (0 %) | |
| Stage 2 – eGFR 60‒89 mL/min | 5 | 3 (60 %) | 2 (40 %) | |
| Stage 3 ‒ eGFR 30‒59 mL/min | 19 | 12 (63.2 %) | 7 (36.8 %) | |
| Stage 4 ‒ eGFR 15‒29 mL/min | 6 | 4 (66.7 %) | 2 (33.3 %) | |
| Stage 5 ‒ eGFR < 15 mL/min | 104 | 45 (43.3 %) | 59 (56.7 %) | |
| Vaccination status | 0.14 | |||
| None | 2184 | 1238 (56.7 %) | 946 (43.3 %) | |
| At least one dose | 419 | 215 (51.3 %) | 204 (48.7 %) | |
| Steroid use | 2051 | 1338 (65.2 %) | 713 (34.8 %) | <0.001 |
| Secondary infection | 428 | 387 (90.4 %) | 41 (9.6 %) | <0.001 |
| Overall mortality | 520 | 428 (82.3 %) | 92 (17.7 %) | <0.001 |
From all admitted patients in third wave with at least one COVID-19 vaccine, 60 % required ICU admission, 36 % IMV and 21 % had a fatal outcome.
DiscussionThis study described the COVID-19 dynamics among hospitalized patients across four consecutive waves from 2020 to 2022, observing the most significant peaks in July 2020, December 2020, June 2021, and January 2022. There were differences in age, chronic diseases frequency, ICU admission, IMV requirement, and LOS among waves. In contrast, there were no differences in overall mortality, which may be explained by the unavailability of new specific drugs against SARS-CoV2 in Colombia during the study period and an incomplete coverage of vaccination in Colombia at the end of the fourth wave. When sub analysis were performed, some differences were observed in the proportion of mortality according to age groups and between ICU and non-ICU patients.
In this study, we observed a marked increase of the cases during the third wave, which can be explained by the emergence of SARS-CoV-2 variants such as gamma and alpha variants circulating by June 2021 concurrently with the third wave of the pandemic in Colombia.11 In the fourth wave, the BA.1.x-Omicron variant predominated; however, there was no significant increase in cases despite the most virulent variant circulating. The higher proportion of vaccinated individuals and the lower association of severe cases with this lineage could explain this observation. These variants have increased transmissibility, virulence, and reduced vaccine efficacy as demonstrated in previous studies.12
Despite the increase in cases in the third wave, the length hospital stays, admission to ICU, and the requirement for IMV were lower than in the first wave. Likewise, mortality was maintained concerning previous waves, and the younger population predominated. Possible underlying causes of these changing patterns are the lower exposure to infection among older individuals, since the youngest was the first to rejoin work and social activities and has been associated with better clinical outcomes respect to the other age groups.13,14
In the fourth wave, there was a substantial decrease in COVID-19 cases. However, it is noteworthy that the most affected population were older than 70 years old since it was expected that the cases in this population would be lower because of being the first to receive the vaccination and boosters. Arregocés-Castillo et al. observed that the effectiveness of vaccines declined with increasing age, probably related to the greater probability of pre-existing conditions in older people, in conjunction with age-related frailty and immunosenescence.15
One of the findings from our study was that, by the end of the fourth wave, vaccination coverage was below 60 %, and among patients admitted to the ICU in the third wave, more than 80 % were not immunized against SARS-CoV-2. Upon comparison, there were no significant differences in vaccination status between ICU and non-ICU patients. Similarly, other studies conducted around the same time reported that approximately 88 % of ICU-admitted patients were unvaccinated.16 Despite the suboptimal vaccination coverage, there was an increase in vaccination rates in comparison to previous waves, which appeared to correlate with a decreased demand for ICU and mechanical ventilation during this period. The low vaccination rates at this point can be explained by various sociocultural and geopolitical factors unique to Colombia at that stage of the pandemic. These include significant delays in vaccine delivery at the pandemic's onset, logistical and socio-political challenges, including protests that disrupted vaccination efforts, and an inclusive yet ambitious strategy to vaccinate the extensive Venezuelan migrant population, which was ineffective in achieving the intended coverage by this point.17
Overall, we observed an improvement in outcomes for patients admitted during the second, third and fourth wave than in the first wave, with a reduction in ICU admission, IMV requirements, and shorter Length of Stay (LOS), similarly to the reported by Fluck et al.18 and Zuil et al.2 but contrary to finding in another study which showed a more severe second wave than the first one in Brazil regarding ICU admission and IMV requirement.3 These differences in severity could be secondary to the new clinical protocols and the experience gained from the first two waves, and the positive effects of immunization. Although we found a higher proportion of patients admitted to the ICU than in other studies,2,19 mortality among hospitalized patients was similar, which may be an indicator of appropriate interventions in patients who required ICU in our institution.
In our study population no patients receive specific therapies approved for COVID-19. Despite several clinical trials and studies have suggested using antivirals such as remdesivir, molnupiravir, and Nirmatrelvir-Ritonavirm, as they have had an impact on mortality in patients with COVID-19.20–22 It was not until March 2022 that molnupilavir was approved for use in Colombia for unvaccinated individuals with no history of COVID-19 infection and risk factors for progression to severe disease.23 This could be one of the factors that explain the lack of variation along the waves in mortality in our population since.
Corticosteroids started to be used in severe cases of COVID-19 for ARDS, given the findings reported by the RECOVERY study.24 This type of medication has been implied in important pathological processes as viral clearance and the modulation of the immune system functions, which are critical for the worsening or recovery of the critically ill COVID-19 patients depending on the therapy timely and dosage.25 De Bruyn et al.26 also found a cumulative dose of corticosteroids administered in the ICU and male sex as risk factors associated with the acquisition of secondary infection. In our study, higher use of corticosteroids was observed among patients who developed secondary infections, mainly in the first wave. However, there was a lack of evidence regarding corticosteroids adequate dosages at the beginning of the pandemic.4,27,28
A major finding in this cohort was that IMV requirement was lower than ICU admission. IMV requirements in our population may reflect the implementation of alternative oxygen therapy strategies such as High-flow nasal cannula, where high-flow oxygen through a nasal cannula decreased the need for mechanical ventilation support and clinical recovery time.29
This study has some limitations. Data were collected from clinical record, thus there could be missing data due to the accuracy and quality of data collection. This study is a single-center study, which may limit external validity and have a potential selection bias. Although we attempted to use national public data to understand infection and reinfection dynamics in our study as an approximation of an ecological study, we do not know exactly the impact of new variants of SARS-CoV-2 and vaccination in our population. In this study data regarding the onset, duration and dosages of steroids were not evaluated. Nonetheless, we believe that this study may represent the region's situation because our hospital receives patients from broad coverage in the country; no similar studies were found locally. The information on the fifth wave is not available, since it was not collected in the registry.
ConclusionsDuring the first four waves of the pandemic, there were differences in the characteristics of patients hospitalized with COVID-19. The potential appearance of SARS-CoV-2 serotypes showed a variation in the number of cases, but not with an increase in mortality, suggesting greater transmissibility but not greater virulence. Vaccination and the experience gained by health professionals in managing patients with SARS-CoV-2 have led to improved clinical outcomes. Efforts are needed to maintain the prevention of the spread of SARS-CoV-2, understand the risk of the new variants, and the effects of available vaccines.
Ethical approval statementThis study was approved by the Comité de Ética en Investigación Biomédica at Fundación Valle de Lili (Approval number: IRB/EC nº 1566). It followed the ethical principles for clinical research by the Declaration of Helsinki. As research without risk according to Colombian law, it was considered exempt from applying informed consent (Resolution 8430 of 1993).
Declaration of generative AI and AI-assisted technologies in the writing processDuring the preparation of this work the authors used Chat-GPT in order to improve English writing. After using this tool/service, the authors reviewed and edited the content as needed and takes full responsibility for the content of the publication.