Human T-lymphotropic virus type 1 (HTLV-1) is a human retrovirus related to the chronic neuroinflammatory disease HTLV-1-associated myelopathy/tropical spastic paraparesis (HAM/TSP). CD4+ T cells activation appears to play a key role on HTLV-1 infection. Here we investigated the expression of genes associated to T cell activation CD3e molecule, epsilon (CD3¿), lymphocyte-specific protein tyrosine kinase (LCK), vav 1 guanine nucleotide exchange factor (VAV1), and zeta-chain (TCR) associated protein kinase 70kDa (ZAP70) on T lymphocytes of HTLV-1-infected individuals and compared to healthy uninfected individuals (CT). We observed that CD3¿, LCK, ZAP70, and VAV1 gene expression were increased in CD4+ T cells from HAM/TSP group compared to HTLV-1 asymptomatic patients (HAC). Moreover, ZAP70 and VAV1 were also upregulated in HAM/TSP compared to CT group. We detected a positive correlation among all these genes. We also observed that CD3¿, LCK, and VAV1 genes had a positive correlation with the proviral load (PVL) and Tax expression. These results suggest that PVL and Tax protein could drive CD3¿, LCK, and VAV1 gene expression in CD4+ T cells, and these genes function on a synchronized way on the CD4+ T cell activation. The elucidation of the mechanisms underlying T cell receptor signaling pathway is of considerable interest and might lead to new insights into the mechanism of HAM/TSP.
Human T-lymphotropic virus type 1 (HTLV-1) is a retrovirus associated with chronic, persistent infection of human T cells. HTLV-1 can infect a wide range of human and nonhuman cells in vitro. However, HTLV-1 preferentially infects CD4+ T cells, which become the main reservoir of HTLV-1.1 HTLV-1 infection is associated with a variety of human diseases, including an aggressive mature T cell malignancy termed adult T-cell leukemia (ATL),2 which is defined as neoplastic growth of HTLV-1-infected T cells. Moreover, HTLV-1 is also associated with non-neoplastic inflammatory conditions such as HTLV-1-associated myelopathy/tropical spastic paraparesis (HAM/TSP).3,4
HAM/TSP is a chronic neuroinflammatory disease characterized by spastic paraparesis, lower limb sensory disturbance, and bladder/bowel dysfunction.5 Although HAM/TSP disorders have been extensively studied, the exact mechanisms by which HTLV-1 induces these inflammatory conditions remain not fully understood. The proviral load of HTLV-1 may contribute to HTLV-1-associated inflammatory conditions development, once the number of HTLV-1-infected T cells circulating in the peripheral blood is higher in patients with HAM/TSP than in asymptomatic HTLV-1-infected individuals.6,7 In addition, increased viral expression, particularly of the transactivating viral gene encoding HTLV-1 Tax protein, has also been hypothesized to play a role on the HTLV-1 disease progression.7,8
Furthermore, many studies considered that differences in gene expression profiles are important insight about the HAM/TSP genesis and might provide potential targeted therapies. In attempt to elucidate the mechanism involved on the HAM/TSP development, our group published a study reporting the gene expression profile in CD4+ T cell isolated from HTLV-1-infected individuals (HAM/TSP and asymptomatic HTLV-1 carriers (HAC)) and healthy individuals (CT) using microarray technology.9 The analysis showed a dendrogram with a clear separation between CT and HTLV-1-infected individuals. We also observed that the HAC and HAM/TSP groups clustered apart. Moreover, we reported some genes involved with HAM/TSP development. These data suggest that HAM/TSP individuals have a different gene expression profile and the differently expressed genes may contribute to the progression of this pathogenesis.
Our group also studied the gene expression profile in CD8+ T cell isolated from HTLV-1-infected individuals.10 We observed a significantly higher expression level of tyrosine kinase ζ chain-associated protein kinase of 70kD (ZAP70), a protein associated with T cell receptor (TCR), in HTLV-1-infected individual compared to the healthy group, suggesting that CD8+ T cells might be activated in the HTLV-1 infection. The role of ZAP70 in HTLV-1 infection is still unknown and there is no data about the ZAP70 expression in CD4+ T cells. Therefore, the aim of this study was to evaluate the TCR Signaling Pathway status of CD4+ T lymphocytes from HTLV-1-infected individuals.
Material and methodsSubjects and cellsA total of 65 individuals were studied: 17 patients with HAM/TSP, 23 asymptomatic HTLV-1 carriers (HAC), and 25 uninfected controls (CT), with fully informed consent. Leukocyte global counts and CD4/CD8T cell ratio were performed in all individuals. Samples of HTLV-1-infected individuals were recruited from the Neurology Department of Clinical Hospital of the Medical School at the University of São Paulo, Ribeirão Preto, São Paulo, Brazil. Inclusion criteria for this study were HTLV-1 positivity by enzyme immunoassay (rp21e-enhanced EIA; Cambridge Biotech) and molecular test (conventional LTR and TAX PCR). All HTLV-1-infected individuals were evaluated for clinical status according to the previously described criteria for ATL and HAM/TSP.11 Individuals from CT group were recruited from the Regional Blood Center of Ribeirão Preto, São Paulo, Brazil. Both HTLV-1-infected and CT individuals were serologically negative for Hepatitis B virus, Hepatitis C virus, Human Immunodeficiency virus, Chagas disease, and syphilis. This study was approved by the Institutional Ethic Committee (process number 3083/2007).
Molecular diagnosis and proviral load measurementThe DNA was extracted from the buffy coat from 17 HAC individuals and 17 HAM/TSP patients using the Super Quick Gene DNA isolation kit (Analytical Genetic Testing Center – AGTC, Denver, CO, USA) following the manufacturer's instructions. The in house PCR (LTR and TAX regions) was performed as previously described.12 The proviral load was quantified using TaqMan®Universal PCR Master Mix (Applied Biosystems, Foster City, CA, USA). The primers sequences were formerly reported.9 Real-time PCR was performed in duplicate for all DNA standards and samples using the ABI Prism 7500 Sequence Detection System (Applied Biosystems, Foster City, CA, USA). The amplification program consisted of 50°C for 2min; 95°C for 10min; followed by 40 cycles at 95°C for 15s/60°C for 1min. The HTLV-1 proviral load value was calculated using the following formula: (copy number of TAX)/(copy number of β-actin)×2×100.000.
CD4+ T cells separation and RNA processingPeripheral blood mononuclear cells (PBMCs) were isolated from whole blood using a Ficoll Hypaque™ PLUS (GE Healthcare Bio-Sciences AB, Uppsala, Sweden). CD4+ cells were positively selected on a MACS column (Miltenyi Biotec, Germany) using magnetic microbeads according to the manufacturer's instructions. CD3+CD4+ T cells purity was confirmed by flow cytometry using the anti-CD4-FITC and anti-CD3-PE (FACSCalibur) (Becton & Dickinson, San Jose, CA, USA) surface markers. The total RNA was isolated with TRIzol®Reagent (Invitrogen, Carlsbad, CA, USA). The RNA was reverse transcribed using a High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA, USA), following the manufacturer's instructions.
Quantitative real-time PCRThe gene expression was analyzed by real-time PCR technique using TaqMan®Gene Expression Assays (Applied Biosystems, Foster City, CA, USA). The PCR amplification and fluorescence data collection were performed with ABI 7500 Sequence Detection (Applied Biosystems, Foster City, CA, USA).
The following genes were studied: CD3e molecule, epsilon (CD3-TCR complex) (CD3¿) (Hs99999153_m1), vav 1 guanine nucleotide exchange factor (VAV1) (Hs00232108_m1), zeta-chain (TCR) associated protein kinase 70kDa (ZAP70) (Hs_00896347), and lymphocyte-specific protein tyrosine kinase (LCK) (Hs00178427_m1). The geometric average of four housekeeping genes human β-actin (ACTB) (4326315E), glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (4310884-E), beta-2-microglobulin (B2M) (4333766-0710013), and ribosomal protein L13a (RPL13A) (185720330-7)13 was used to normalize the mRNA amount for each sample (Applied Biosystems). The 2−ΔΔCT method was chosen to calculate the relative expression levels.14 All these experiments were performed in duplicates.
Tax expression analysisThe Tax protein expression was evaluated in 11 HAC individuals and 9 HAM/TSP patients. To detect Tax protein expression in HTLV-1-infected cells, the whole PBMCs were cultured in RPMI 1640 (Sigma-Aldrich, Saint Louis, MO, USA) containing 10% fetal bovine serum (HyClone, Logan, UT, USA) and 20nM concanamycin A (Sigma-Aldrich, Saint Louis, MO, USA), a potent cytolysis inhibitor of CD8+ T cell that allows the CD4+ T cell survival. The cells were incubated for 12h under 5% CO2 at 37°C. Then, the cells were stained for the surface markers anti-CD4-PE, anti-CD8-PerCP, and anti-CD3-APC (Becton & Dickinson, San Jose, CA, USA). For intracellular analysis, cells were fixed with 4% paraformaldehyde in phosphate-buffered saline (PBS) for 20min. After this time, the fixed cells were washed with PBS containing 4% of normal goat serum (NGS) (Sigma-Aldrich, Saint Louis, MO, USA) and then washed with PBS containing 0.1% Triton X-100 (Sigma-Aldrich, Saint Louis, MO, USA) for 10min at room temperature. Permeabilized cells were washed and resuspended in PBS/4% NGS containing isotype control mAb (Southern Biotechnology Associates, Birmingham, AL) or anti-HTLV-I Tax mAb (Lt-4; IgG3), provided by Dr. Yuetsu Tanaka, University of the Ryukyus, Okinawa. Finally, the cells were incubated for 30min at room temperature and were stained intracellularly with Alexa Fluor 488-labeled goat anti-mouse IgG3 (Invitrogen, Carlsbad, CA, USA) for 30min at room temperature. All samples were analyzed on a FACSCalibur flow cytometer (Becton & Dickinson, San Jose, CA, USA) and 10,000 total events were acquired.
Statistical analysisComparisons among groups were performed using analysis of variance (ANOVA) followed by Tukey test. Correlation among different parameters was evaluated using the Spearman's Rank Correlation. Data regarding gender and age were analyzed using the Fisher's exact test. Critical p-values were considered statistically significant if below 0.05. Data sets were compiled and analyzed in GraphPad PRISM, version 5.01 (GraphPad software, CA, USA).
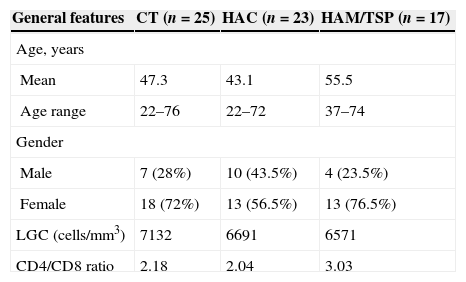
ResultsClinical features of HTLV-1-infected individualThe clinical features of the studied population are shown in Table 1. The HAM/TSP group consisted of 76.5% women and 23.5% men, mean age 55.5 years, age range 37–74 years. In the HAC group there were 56.5% of women and 43.5% of men, mean age 43.1 years, age range 22–72 years. No significant gender difference was observed between HAC and HAM/TSP groups, whereas age was significantly different (p=0.0053).
General features of healthy controls and HTLV-1 infected individuals (HAC and HAM/TSP) according to age, gender, leukocytes global count and CD4/CD8 ratio.
| General features | CT (n=25) | HAC (n=23) | HAM/TSP (n=17) |
|---|---|---|---|
| Age, years | |||
| Mean | 47.3 | 43.1 | 55.5 |
| Age range | 22–76 | 22–72 | 37–74 |
| Gender | |||
| Male | 7 (28%) | 10 (43.5%) | 4 (23.5%) |
| Female | 18 (72%) | 13 (56.5%) | 13 (76.5%) |
| LGC (cells/mm3) | 7132 | 6691 | 6571 |
| CD4/CD8 ratio | 2.18 | 2.04 | 3.03 |
CT, healthy controls; HAC, asymptomatic HTLV-1 carrier; HAM/TSP, HTLV-1 associated mielopathy/tropical spastic paraparesis; LGC, leukocytes global count.
The CT group was composed of 72% women and 28% men, mean age 47.3 years, age range 22–76 years. HAM/TSP group had higher CD4/CD8T cell ratio than CT and HAC groups, however, this difference was not significant; no difference was observed in leukocyte global counts among groups.
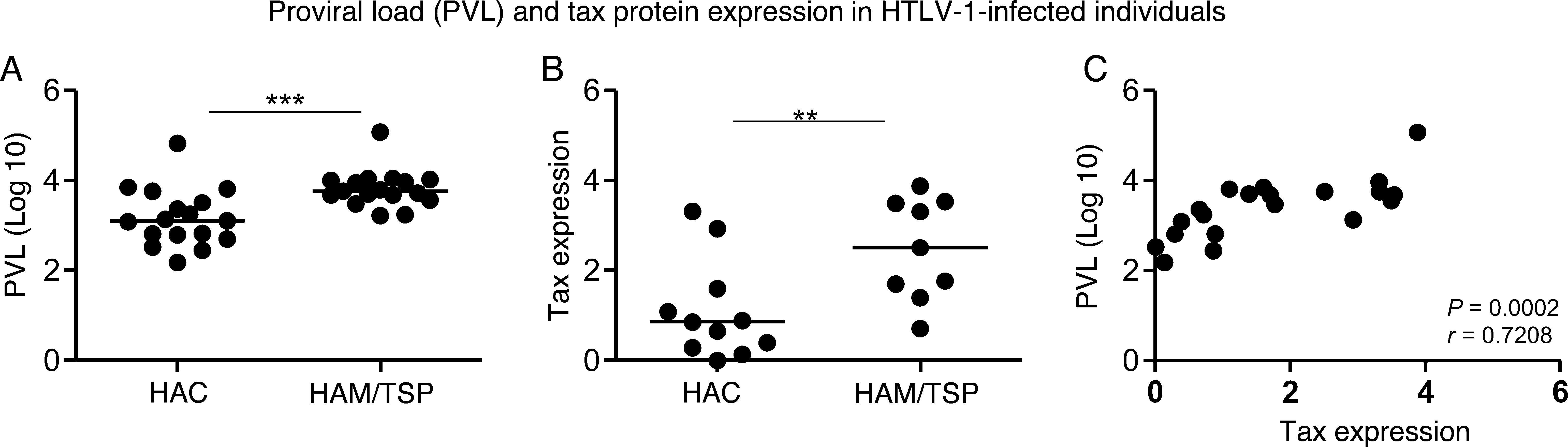
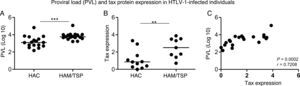
Both Tax expression in CD4+ T cells and proviral load were increased in the HAM/TSP group (p=0.0007 and p=0.0061, respectively) when compared to the HAC group (Fig. 1A and B). Moreover, proviral load positively correlated to Tax expression (r=0.7208; p=0.0002) (Fig. 1C).
Proviral load (PVL) and Tax protein expression (percentage of Tax+CD3+CD4+ T cells) measurement in HTLV-1-infected individuals. (A) Proviral load was measured in HAC (n=17) and HAM/TSP (n=17) individuals. (B) Tax protein expression was quantified in HAC (n=11) and HAM/TSP (n=9) individuals. The Mann–Whitney U test was used to evaluate differences among groups (**p≤0.01; ***p≤0.001). (C) Correlation between PVL and Tax expression in HTLV-1-infected individuals (n=20). Correlation was calculated by the Spearman method. HAC: Asymptomatic HTLV-1 carrier; HAM/TSP: HTLV-1 associated mielopathy/tropical spastic paraparesis.
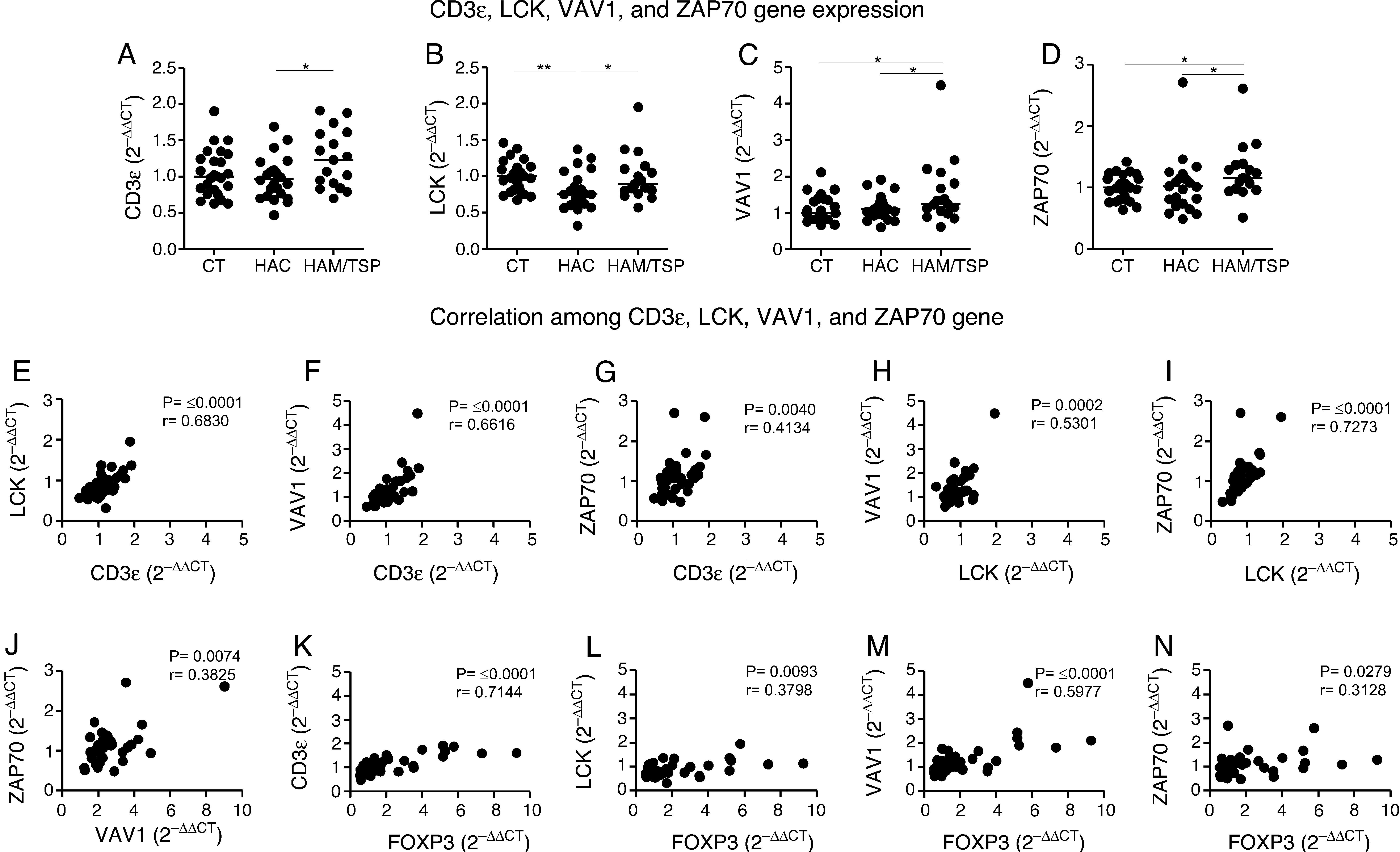
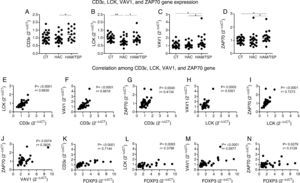
The levels of CD3¿, LCK, VAV1, and ZAP70 gene expression were evaluated by quantitative real-time PCR (Fig. 2A–D). We observed that all these genes were significantly overexpressed in HAM/TSP group compared to HAC group. Additionally, VAV1 and ZAP70 genes were increased in HAM/TSP group compared to CT group (p=0.0364 and p=0.0120, respectively).
CD3¿, LCK, VAV1, and ZAP70 genes expression by real-time PCR and correlation among them. Gene expression levels comparison of CD3¿ (A), LCK (B), VAV1 (C), and ZAP70 (D) among CT (n=25), HAC (n=23), and HAM/TSP (n=17) groups. The Mann–Whitney U test was used to evaluate differences among groups (*p≤0.05; **p≤0.01). A positive correlation between LCK and CD3¿ (E), VAV1 and CD3¿ (F), ZAP70 and CD3¿ (G), VAV1 and LCK (H), ZAP70 and LCK (I), ZAP70 and VAV1 (J), CD3¿ and FOXP3 (K), LCK and FOXP3 (L), VAV1 and FOXP3 (M), and ZAP70 and FOXP3 (N) was observed in the samples from HTLV-1-infected individuals. Correlation was calculated by the Spearman method. CT: Healthy control; HAC: Asymptomatic HTLV-1 carrier; HAM/TSP: HTLV-1 associated mielopathy/tropical spastic paraparesis.
In order to determine the activation of T cell receptor signaling pathway, we studied the correlation among CD3¿, LCK, VAV1, and ZAP70 genes in HTLV-1-infected individuals. We observed a positive correlation among all these genes (Fig. 2E–J). Correlations among genes were also performed in HAC and HAM/TSP groups individually. Both groups revealed a positive correlation between most of the genes, except for the correlation between ZAP70 and CD3¿ genes in HAC group, and ZAP70 and VAV1 genes in both groups (data not shown). In this individual analysis of genes correlation, we observed that HAM/TSP group showed a more significant genes correlation compared to HAC group (data not shown).
Since the regulatory T-cells (Treg) are related to CD4+ T-cells in HTLV-1-infected population,15 we believe that FOXP3, one of the main Treg cell markers,16 might be associated to the genes of T cell receptor signaling pathway. In a recent study9 we reported that FOXP3 gene expression was upregulated in HAM/TSP group compared to the CT and HAC groups. Moreover, the percentage of CD4+FOXP3+ cells was increased in HTLV-1-infected individuals compared to CT group. Therefore, we performed the correlation between FOXP3 gene expression and the CD3¿, LCK, VAV1, and ZAP70 genes in HTLV-1-infected individual. This correlation was based on the FOXP3 results obtained in our previously study.9 A positive correlation was observed between FOXP3 and all the analyzed genes (Fig. 2K–N).
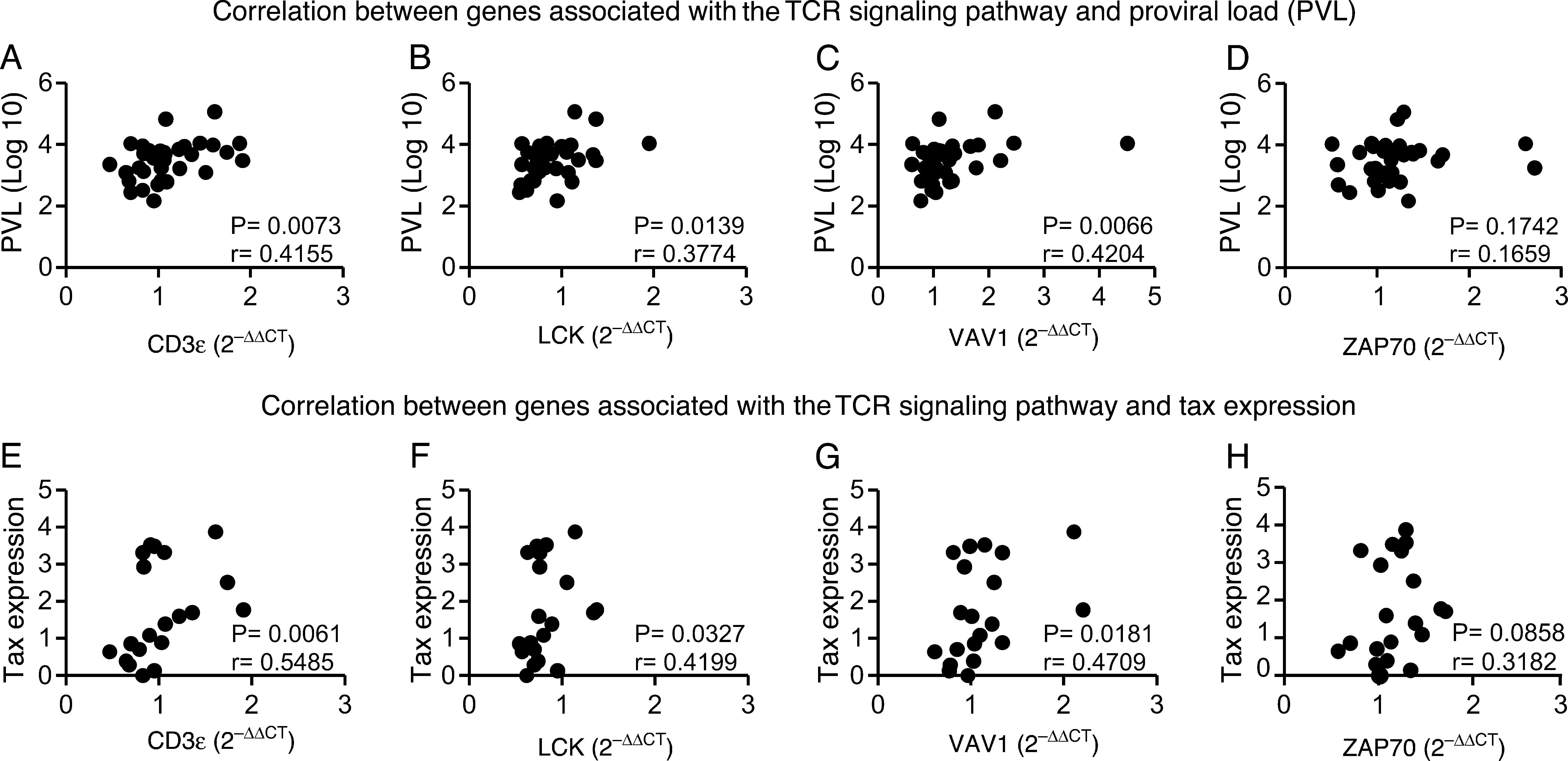
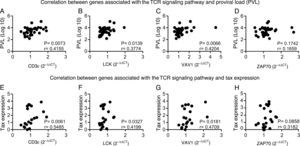
CD3¿, LCK, VAV1 are correlated to PVL and Tax expressionSince CD3¿, LCK, VAV1, and ZAP70 genes were upregulated in HAM/TSP compared to HAC group; we suggested that these genes could be correlated to the PVL and Tax expression. We performed Spearman's correlation test and verified that CD3¿, LCK, and VAV1 genes were positively correlated to plasma viral load and Tax expression in HTLV-infected samples, while ZAP70 gene did correlate neither with plasma viral load nor with Tax expression (Fig. 3A–H).
Correlation between genes expression of CD3¿, LCK, VAV1, and ZAP70, and percentage of Tax expression or proviral load (PVL) in HTLV-1-infected individuals. A positive correlation between PVL and CD3¿ (A), LCK (B), and VAV1 (C) was observed. (D) Correlation between PVL and ZAP70. The Tax expression positively correlated to CD3¿ (E), LCK (F), and VAV1 (G). H) Correlation between Tax expression and ZAP70. Correlation was calculated by the Spearman method.
We have shown, in our previous study, which the ZAP70 gene was overexpressed in CD8+ T cells from HTLV-1-infected individuals when compared to uninfected controls. The CD4+ T cells gene expression profile has been extensively studied in HTLV-1-infected individual because these cells are preferentially infected by HTLV-1. Therefore, the CD4+ T cells are the main reservoir of HTLV-1.1 Accordingly, here we evaluated the gene expression of genes associated with the TCR Signaling Pathway in CD4+ T lymphocytes of HTLV-1-infected individuals. We observed that CD3¿, LCK, ZAP70, and VAV1 gene expression were increased in CD4+ T cells from HAM/TSP group. Moreover, we detected a positive correlation among all these genes.
ZAP70 is a protein tyrosine kinase (PTK) that can control cytoskeleton modifications, adhesion, mobility, and activation of T lymphocytes.17–20 The engagement of the TCR/CD3 complex induces a series of intracellular events that culminates in the activation of PTKs. The TCR signal transduction involves four families of PTKs: Csk kinase, Src kinase, Tec kinase, and the ZAP70 kinase.21 After TCR engagement, Src kinase lymphocyte kinase (LCK) is activated and its kinase domain engages the phosphorylation of CD3 ITAMs, notably on the ζ chain. Consequently, ZAP70 is recruited, phosphorylated and it can associate with different molecules of the TCR/CD3 complex such as CD3 ζ, δ, and ¿ chains. Activated ZAP-70 phosphorylates a number of downstream adaptors, including VAV1. All this activation and interaction with individual components of the TCR play a central role in the T lymphocytes activation.22–26
It was described that some virual infections are associated with selective TCR/CD3 activation defects, such as Epstein–Barr virus (EBV),27 cytomegalovirus (CMV),28 and human immunodeficiency virus (HIV).29 Among the immunologic defects associated with HIV-1 infections are lymphopenia and low lymphocyte proliferative responses after stimulation with antigen or mitogens.30,31 Deficiency of TCR/CD3-directed CD4+ T cell immune responses is often observed in individuals infected with HIV.32 Additionally, CD4+ T cell proliferation deficiency is associated with an impaired CD3-induced tyrosine phosphorylation proccess.33,34 It was reported that the TCR/CD3 signaling chain CD3ζ, but not CD3¿, was significantly decreased in T cells from AIDS patients by flow cytometry.35,36 Consequently, an impaired TCR/CD3 activation in HIV infection can be related to CD4+ T cell proliferation deficiency.
A study showed that ZAP-70 is absent in three HTLV-1-infected cells lines (C8166, MT-2, and ATL-2). Besides, it was investigated the role played by Tax protein in the downregulation of ZAP-70 by using Jurkat T cells that stably expressed Tax and it was observed that Tax induces a downregulation of ZAP-70 expression.37 In contrast to these results, we found that CD3¿, LCK, ZAP70, and VAV1 gene expression were increased in CD4+ T cells from HAM/TSP group, indicating activation of TCR signaling pathways. These divergent results could be due to the fact that in our study we used HTLV-1-infected individuals cells instead of cultured cells lines.
We have recently reported that FOXP3 gene expression, which is a master regulator gene for the regulatory T (Treg) cells, was significant increased in HAM/TSP group compared to CT and HAC groups. Moreover, a positive correlation was detected between FOXP3 gene and Tax expression and proviral load. The percentage of CD4+FOXP3+ cells was increased in HTLV-1-infected individuals compared to CT group. Based on these results, we hypothesize that the FOXP3 gene could be correlated with T cell receptor signaling pathway genes. The correlation was performed using the FOXP3 results obtained in our previous study.9 A positive correlation between FOXP3 and all the analyzed genes was observed. These data suggest that Treg cells play an important role in HTLV-1 infection. It has been described that FOXP3+ T cells is the main reservoir of HTLV-1 provirus in HAC and HAM/TSP individuals, showing that these cells are in vivo preferential targets of HTLV-1.1,38,39 In addition, FOXP3+ T cells have been recognized to migrate faster to the inflammatory sites, which might facilitate migration and consequently the HTLV-1 infection spread.40
We also observed that CD3¿, LCK, and VAV1 genes had a positive correlation with plasma viral load and Tax expression. Tax is responsible for controlling the expression of viral and cellular genes, including genes involved in T cell proliferation and activation.41,42 Additionally, the risk of HTLV-1-inflammatory diseases are strongly correlated to the proviral load.43 This way, all these results suggest that Tax viral protein could drive CD3¿, LCK, and VAV1 gene expression in CD4+ T cells and these genes function on a synchronized way with T cell activation. Since CD4 T cell receptor signaling pathway is activated, there is exacerbated immunological response and the role of this response in controlling the HTLV-1 infection results in the tissue damage that occurs in HAM/TSP. However, the obtained results were not confirmed on protein level, which is hindered by lack of samples due to the impossibility to perform new recruitment of the tested patients. Furthermore, ZAP70 phosphorylation also could be tested to assess whether this pathway may be contributing to CD4 activation and triggering an exacerbated inflammatory response.
Our data have important biological implications for the understanding of the role of T cell receptor signaling pathway in the HTLV-1 infection. Additionally, the elucidation of the mechanism underlying this pathway might lead to new insights into the HAM/TSP mechanisms.
Conflicts of interestThe authors declare no conflicts of interest.
The authors thank Prof. Charles Bangham for Tax expression analysis. They are also grateful to the patients. This work was supported by Fundação Hemocentro de Ribeirão Preto (FUNDHERP), Centro Regional de Hemoterapia de Ribeirão Preto (CRH), Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq).