Appearance of isolated reports of resistance to anti-methicillin-resistant Staphylococcus aureus (MRSA) drugs is worrisome underscoring the need to continuously monitor the susceptibility of clinical MRSA isolates to these drugs. Hence, the present study is conducted to determine the susceptibility of MRSA isolates to various classes of anti-MRSA drugs such as vancomycin (glycopeptide), daptomycin (lipopeptide), tigecycline (glycylcycline), and linezolid (oxazolidinone) to determine the MIC50 and MIC90 values, and to observe MIC creep over a three year period, if any, with respect to these drugs.
MethodsA total of 200 isolates of MRSA obtained from clinical specimens were included. MIC was determined by E-test for anti-MRSA antibiotics vancomycin, linezolid, daptomycin, and tigecycline. Non-parametric methods (Kruskal–Wallis and Chi-square test) were used to assess MIC trends over time. In addition, MIC50 and MIC90 values were also calculated.
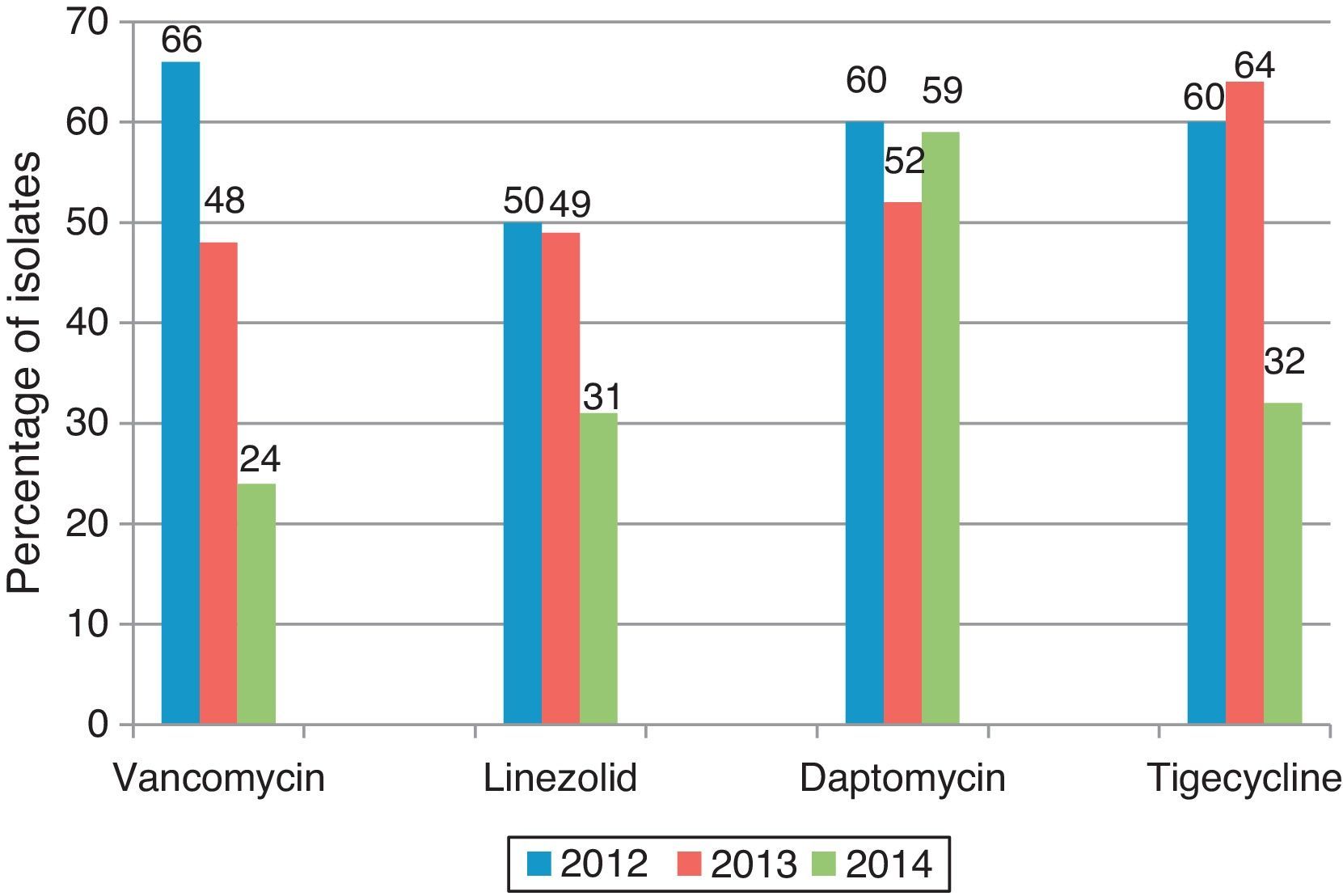
ResultsNo isolate was found resistant to vancomycin, daptomycin, or linezolid; five isolates were resistant to tigecycline. Seven VISA isolates were encountered with the MIC value for vancomycin of 4μg/mL. MIC values for vancomycin, tigecycline, linezolid showed a definite increase over a 3-year period which was statistically significant with p-values <0.0001, 0.0032, 0.0242, respectively. When the percentage of isolates with a median MIC value less than or equal to that of the index year was calculated, the change was most striking with vancomycin. The proportion of isolates with higher MIC values was greater in 2014 than 2012 and 2013.
ConclusionMIC creep was notably observed with vancomycin, and to some extent with tigecycline and linezolid. Selection pressure may result in creeping MICs, which may herald the emergence of resistant organisms.
Vancomycin has been the mainstay of therapy for serious infections caused by methicillin-resistant Staphylococcus aureus (MRSA), because of its relatively good safety profile, its low potential to induce resistance, and for many years, the lack of other approved alternatives.1 However, its efficacy has become uncertain, because of its slow bactericidal activity, the emergence of isolates with reduced susceptibility and possible “MIC creep” among susceptible strains.2
In the last few years, newer antibiotics such as linezolid, daptomycin, tigecycline, dalbavancin, telavancin, oritavancin, ceftobiprole, ceftaroline, and iclaprim have been added to the arsenal of anti-MRSA drugs with many of them already in clinical use and some on the horizon. Linezolid is a synthetic oxazolidinone that inhibits the initiation of protein synthesis at the 50s ribosome and has bacteriostatic activity against MRSA. It is currently approved by the United States Food and Drug Administration (US FDA) for the treatment of complicated skin and skin-structure infections (SSSIs) and nosocomial pneumonia caused by susceptible pathogens, including MRSA.1 Daptomycin is a cyclic lipopeptide that causes depolarization of the bacterial cell membrane and has bactericidal activity. It is recommended for treatment of skin and skin structure infections, bacteremia, and right-sided endocarditis caused by MRSA, as well as patients with prolonged MRSA bacteremia, who are at high risk for metastatic complications and death.3 Tigecycline is the first glycylcycline class of antibiotic which has bacteriostatic activity against MRSA, has been approved by US FDA for the treatment of complicated skin and skin structure infections (cSSSI) and complicated intra-abdominal infections.4 Tigecycline can overcome the two common mechanisms that are associated with tetracycline resistance – ribosomal protection and efflux pumps.5
Appearance of isolated reports of resistance to these alternative drugs is worrisome underscoring the need to continuously monitor the susceptibility of clinical MRSA isolates to these drugs. The present study was conducted to determine the susceptibility of MRSA isolates to various classes of anti-MRSA drugs such as vancomycin (glycopeptide), daptomycin (lipopeptide), tigecycline (glycylcycline) and linezolid (oxazolidinone) to determine the MIC50 and MIC90 values and to observe MIC creep, if any, with respect to these drugs.
Materials and methodsA total of 200 isolates of MRSA obtained from clinical specimens (pus – 119; blood – 12; tissue bit – 9; pleural fluid – 2; tracheal aspirate – 4; wound swab – 40, and 14 from other specimens) submitted to Department of Microbiology, JIPMER, Puducherry from January 2012 to December 2014. The isolates were almost equally distributed over the three-year period – 2012 (62), 2013 (63), and 2014 (75). Only one isolate per patient was included in the analysis. In case of multiple isolates, the first isolate was included. Minimum inhibitory concentration (MIC) was determined by E-test for anti-MRSA antibiotics vancomycin, linezolid, daptomycin, and tigecycline, according to manufacturer's instructions (bioMérieux,) and the results were interpreted as per CLSI guidelines6 and EUCAST guidelines7 for tigecycline. For quality control, Staphylococcus aureus ATCC 29213 was employed. Methicillin resistance was confirmed by PCR for mecA gene.
The percentage of isolates with a median MIC value less than or equal to that of the index year (2012) was calculated for each antibiotic in the three years under study, to observe changes in the proportion of isolates with lower MIC values, which would suggest an “MIC creep”. In addition, MIC50 and MIC90 values of vancomycin, linezolid, daptomycin, and tigecycline were also calculated.
Statistical analysisChi-square test was employed for the assessment of significant change, if any, among the MIC values of isolates in three years in comparison with the median MIC value of the index year (2012). MIC trends over the three years and the significance of changes in MIC values were assessed using non-parametric Kruskal–Wallis test with the help of Graphpad prism 6.0 software. p-Value <0.05 was considered significant.
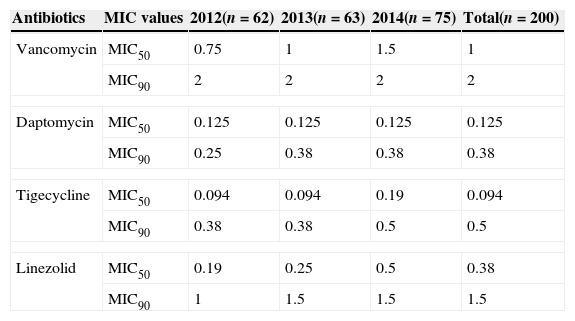
ResultsThe MIC values for vancomycin ranged from 0.125 to 3mg/L, for linezolid from 0.016 to 2mg/L, for daptomycin from 0.016 to 0.5mg/L, and for tigecycline from 0.016 to 1mg/L. MIC50 and MIC90 values are depicted in Table 1.
MIC50 and MIC90 of anti-MRSA drugs.
| Antibiotics | MIC values | 2012(n=62) | 2013(n=63) | 2014(n=75) | Total(n=200) |
|---|---|---|---|---|---|
| Vancomycin | MIC50 | 0.75 | 1 | 1.5 | 1 |
| MIC90 | 2 | 2 | 2 | 2 | |
| Daptomycin | MIC50 | 0.125 | 0.125 | 0.125 | 0.125 |
| MIC90 | 0.25 | 0.38 | 0.38 | 0.38 | |
| Tigecycline | MIC50 | 0.094 | 0.094 | 0.19 | 0.094 |
| MIC90 | 0.38 | 0.38 | 0.5 | 0.5 | |
| Linezolid | MIC50 | 0.19 | 0.25 | 0.5 | 0.38 |
| MIC90 | 1 | 1.5 | 1.5 | 1.5 | |
No isolate was found resistant to vancomycin, daptomycin, or linezolid. However, five isolates had MIC values greater than 0.5mg/L for tigecycline, which is the susceptibility breakpoint for MRSA. Although, no resistance to vancomycin was observed, 9 isolates had the MIC value of 3mg/L, which falls in the range of intermediate susceptibility. For confirmation, agar dilution was also performed and the MIC value was found to be 4mg/L for 7 isolates. According to CLSI guidelines, those isolates were identified as vancomycin-intermediate S. aureus (VISA).
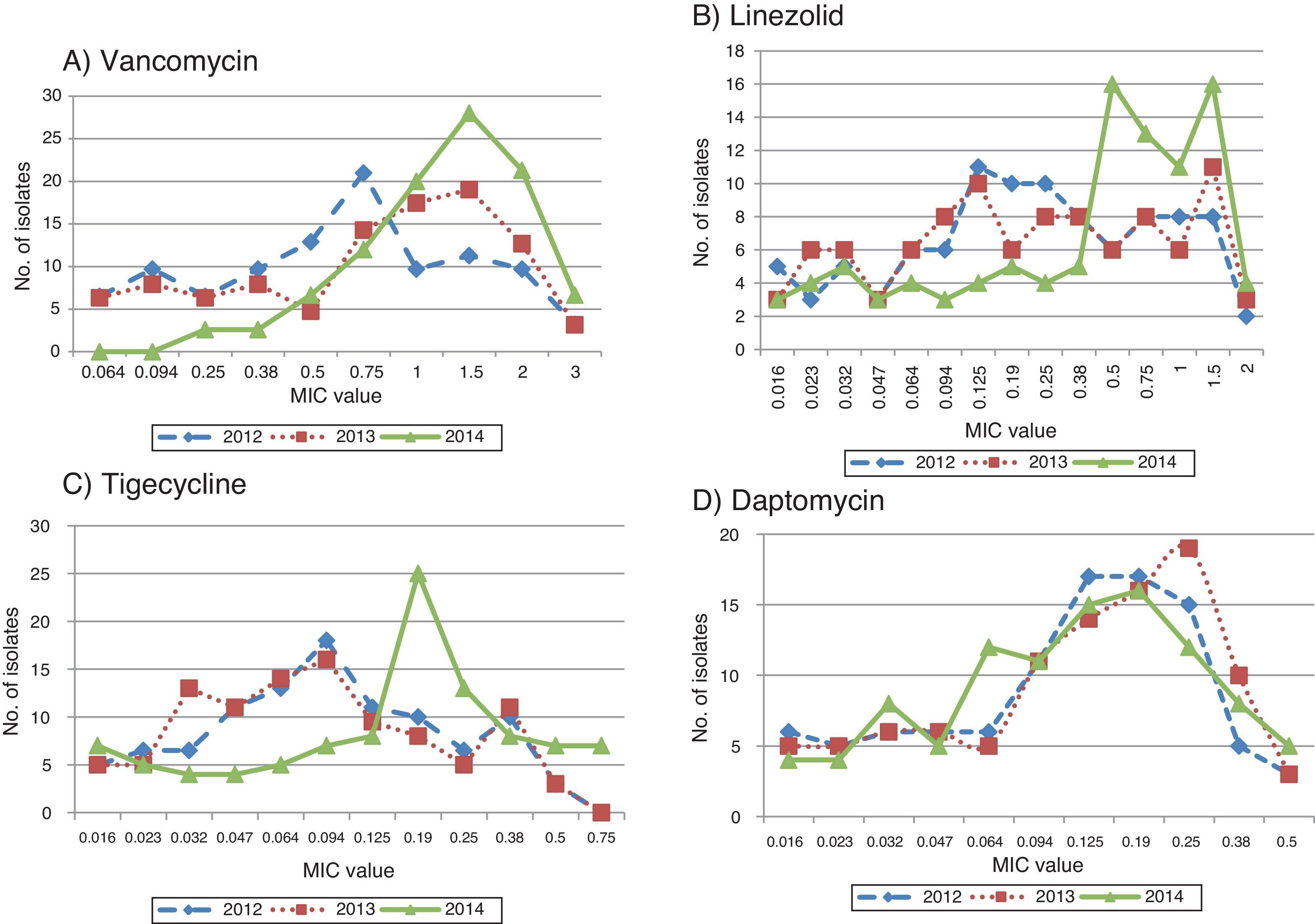
MIC creepMIC values of vancomycin, tigecycline, and linezolid showed a definite increase over a three-year period which was statistically significant with the p-values <0.0001, 0.0032, 0.0242, respectively.
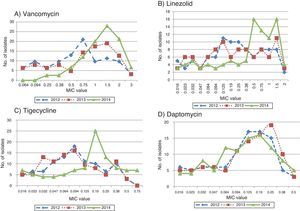
The 2012 median MIC values for vancomycin, linezolid, daptomycin, and tigecycline were 0.75mg/L, 0.19mg/L, 0.125mg/L, and 0.094mg/L, respectively. When the percentage of isolates with a median MIC value less than or equal to that of the index year was calculated, the change was most striking with vancomycin with the proportion being significantly lower in 2014 when compared to the preceding two years, indicating that “MIC creep” had indeed occurred (Fig. 1).
As a further evidence for MIC creep, the proportion of isolates with higher MIC values was greater in 2014 than 2012 and 2013. This was again most notably observed with vancomycin and tigecycline, and to some extent with linezolid (Fig. 2A–C). However, with daptomycin, the proportion of isolates with lower MIC values was greater in 2014 when compared to the previous years (Fig. 2D).
DiscussionFor more than four decades, vancomycin has been the cornerstone of therapy to treat serious infections caused by MRSA, but the emergence of VISA, hVISA and VRSA has compromised its efficacy. From India, only a few studies have reported VISA/VRSA from different regions. An earlier study from our hospital reported a single VISA with MIC 5mg/L, among 102 clinical isolates.8 In a study from Hyderabad, 16 VISA and 7 VRSA were reported out of 358 S. aureus isolates tested.9 The overt presence of VISA/VRSA is only the tip of the iceberg, whereas the greater danger is the phenomenon of MIC creep, which may herald the emergence of resistance compromising the clinical utility of the antibiotic.
Although, none of the VISA isolates were from serious/life threatening infections in the present study (all VISA isolates were from skin and soft tissue infections) the point of concern is that two of them were community acquired, raising the possibility of emergence and spread of VISA isolates outside the hospital setting.
In any given microbial population, the central MIC tendency can increase over time, and the phenomenon is termed as MIC “creep.” This phenomenon is influenced by many factors, wherein clonal replacement and antibiotic exposure can play a vital role. Failure rates exceeding 60% for S. aureus displaying a vancomycin MIC value of 4mg/L prompted CLSI recommendations to lower the breakpoint for susceptibility from 4 to 2mg/L, in 2006.10
The clinical impact of rising MICs of vancomycin could not be accurately assessed, as most of those patients were not treated with vancomycin although the association of increased vancomycin MIC and vancomycin treatment failures has been well documented worldwide. In the last decade, many studies had demonstrated the association between higher vancomycin MICs and poorer clinical outcomes, though a few studies have shown no such association.11 In a meta-analysis, Jacob et al. concluded that “a susceptible but high MIC to vancomycin is associated with increased mortality and treatment failure among patients with MRSA infections”.12 Holmes et al. have demonstrated the association between higher vancomycin MIC and increased mortality in patients with MRSA bacteremia13 and nosocomial pneumonia; Choi et al. have demonstrated slower clinical response and higher relapse rate than patients infected with low vancomycin MIC isolates.14 Similarly, Maclayton et al. have also reported that hemodialysis patients who had MRSA bacteremia with a vancomycin MIC of 2mg/L experienced a longer mean hospital length of stay and increased hospital costs compared with patients with MRSA bacteremia with a vancomycin MIC less than or equal to 0.5mg/L.15
Using categorical representation of data such as percentage susceptible or breakpoint MICs, or even with traditional percentile MIC markers such as MIC50 and MIC90 values, there is a strong likelihood that population shifts in MIC values will go undetected. Conventional CLSI recommended broth dilution method might not be able to detect subtle changes. E-Test, on the other hand, is capable of detecting these changes by incorporating intermediate MIC dilutions.16 Though E-test has gained credibility for MIC determination, higher MICs observed in the present study should be interpreted with caution, as E-test has been reported to give higher MIC values than that of reference broth microdilution method.2 Study reports have shown that E-test provides vancomycin and daptomycin MIC results consistently higher by 0.5–1.5log2 dilutions than those provided by precisely performed reference BMD tests.17
In this study, MIC50 and MIC90 values for vancomycin and daptomycin were found to be 1 and 2mg/L and 0.19 and 0.38mg/L, respectively, which were comparable to the results of the study reported from PGIMER, in 2014.18 MIC90 value of daptomycin has been found a little higher in our study. A recent study from Brazil has reported tigecycline and daptomycin resistance in 10 and 2 MRSA isolates, respectively, out of 36 isolates.19 Five of the MRSA isolates in the present study exhibited higher MIC values for tigecycline above the susceptibility breakpoint (≤0.5mg/L), which is of concern. CLSI recommendations are not available for the susceptibility testing of S. aureus, yet it is recommended by EUCAST. The finding reported here, with reduced susceptibility to tigecycline was therefore considered to be significant.
The usage of vancomycin in our hospital has seen a marked increase in the last decade while linezolid has been in limited use (for skin and soft tissue infections) in the last two years suggesting that selection pressure may be the underlying trigger for the observed MIC creep for vancomycin and to some extent for linezolid. The same may not hold true for daptomycin and tigecycline as they are not a part of our hospital formulary. However, patients’ prior exposure to these antibiotics cannot be ruled out.
None of the MRSA isolates in our study showed resistance to daptomycin and linezolid. Daptomycin has been studied by a number of investigators and has proved to be effective for bacteremic infections due to staphylococci as well as vancomycin-resistant enterococci and other Gram-positive organisms.20 It has been hypothesized that antibiotics which inhibit protein synthesis may be effective against susceptible toxin-producing strains. Recently, it has been found in an in vitro model that linezolid, along with clindamycin, reduce the production of Panton-Valentine leukocidin toxin, alpha-hemolysin, and toxic-shock syndrome toxin-1.21 Hence, treatment with linezolid might have an added advantage in treating skin and soft-tissue infections caused by CA-MRSA, which are toxin producers.
Although, no alarming rates of resistance to anti-MRSA drugs was observed in our study, we should guard against complacence as a combination of selection pressure and creeping MIC may result in the emergence of resistant organisms. This study underscores the need for continuously monitoring the shifts in MIC values even in the susceptible range. A limitation of the study was the lack of data on clonality of isolates by molecular typing methods such as PFGE or MLST. This would have shed light on the spread of a particular clone with higher vancomycin and linezolid MICs.
Author's contribution- 1.
Niveditha N – carried out the work and was involved in drafting the manuscript.
- 2.
Sujatha S – designed and supervised the work and was involved in critical revision of the manuscript.
The authors declare no conflicts of interest.