Community-associated methicillin-resistant Staphylococcus aureus (CA-MRSA) are increasingly causing infective endocarditis over the past decade. Here we report a healthy man who developed a severe acute infective endocarditis with systemic embolism caused by CA-MRSA. The strain was recovered from repeated blood cultures and was characterized using molecular detection and genotyping. The S. aureus isolate was typed as ST630 SCCmecV with spa-type t4549, agrI/IV and was PVL-negative. This is the only case report, to our knowledge, of CA-MRSA infective endocarditis in China. This case highlights the emergence and geographical spread of life-threatening CA-MRSA infection within China.
Since the first genuine community-associated methicillin-resistant Staphylococcus aureus (CA-MRSA) infection was reported in Australia in the early 1990s, the emergence of CA-MRSA infection has increased in recent years all over the world. CA-MRSA is primarily associated with healthy individual skin and soft tissue infections. Additionally, invasive diseases, including bacteremia, infective endocarditis (IE), osteomyelitis, and hemorrhagic necrotizing pneumonia, also have been reported. Infective endocarditis can seriously damage heart valves and cause other serious complications, associated with significant morbidity and mortality, and it is lethal if not promptly treated with appropriate antibiotics, regardless of whether surgery is performed.1 However, despite improved diagnostic techniques and advances in treatment options, neither its incidence nor mortality has decreased in the past years.2S. aureus is a leading cause of left-sided infective endocarditis in developing countries, nevertheless, only sporadic cases of endocarditis caused by CA-MRSA among healthy individuals have been reported.3 Here we describe the first case of infective endocarditis complicated with systemic embolism due to a novel CA-MRSA ST630 in China, which was successfully treated with a combination of antimicrobials and surgical therapy.
Case presentationA 49-year-old previously healthy man was admitted to the hospital with a 10-day history of chills, fever, and dyspnea in August 2011. He had no history of surgery or intravenous drug use, and had no notable medical record. At the time of hospital admission, physical examination revealed a pulse rate of 62 beats per min, respiratory rate of 20 breaths per min, and blood pressure of 125/83mmHg. A systolic murmur was best heard at the apex. He was febrile (40.7°C) and he had a minor abrasion on left foot. The rest of his physical examination was unremarkable. Pertinent laboratory investigations revealed white blood cell count of 23,100/mm3, with 94.5% neutrophils, 3.2% lymphocytes, and 2.3% mononuclear cells, platelets of 40,000/mm3 and C-reactive protein of 58.6mg/L. The patient was hospitalized for a presumptive diagnosis of septicemia. Empirical antibiotics were started with vancomycin and levofloxacin (0.5g q8h) therapy.
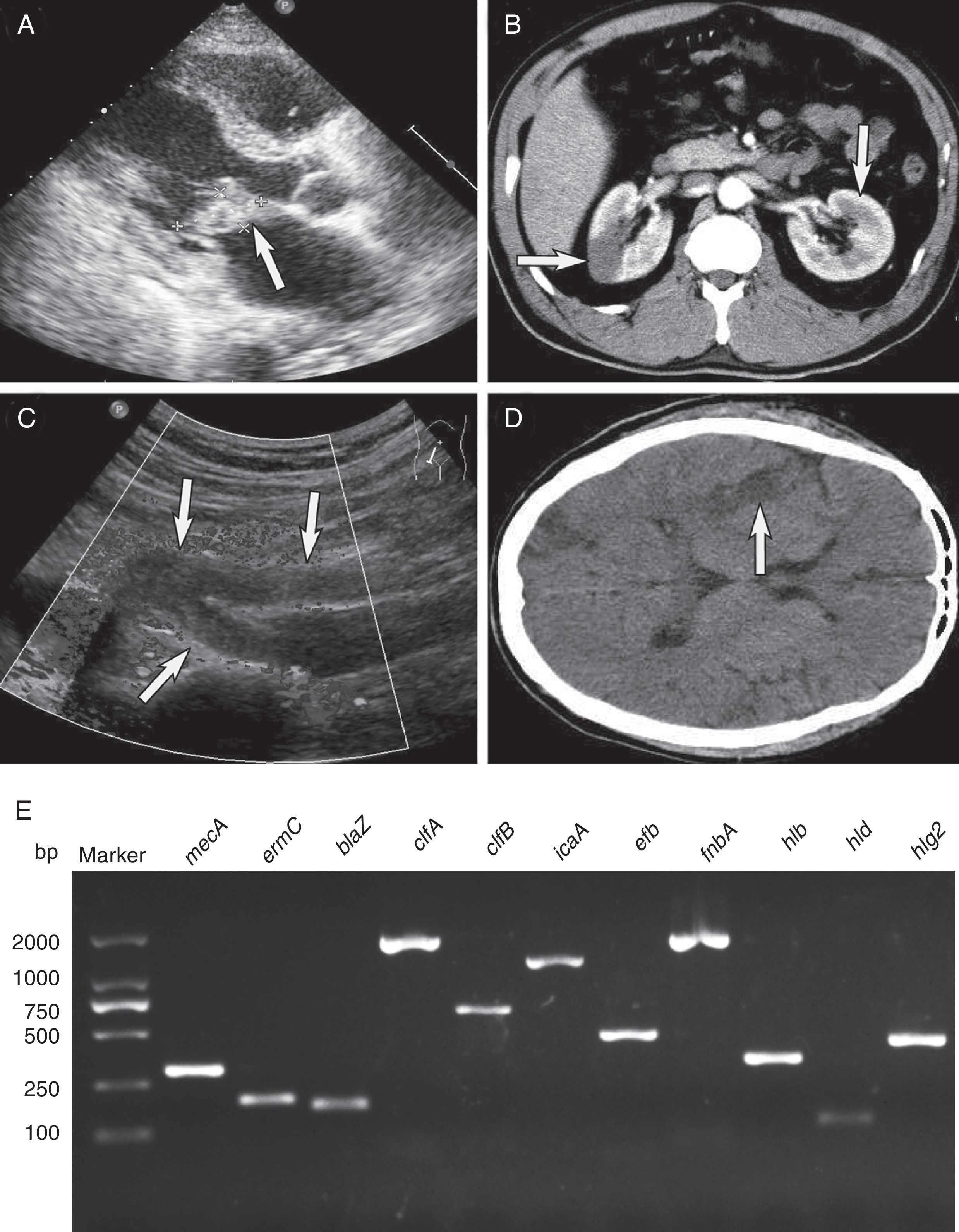
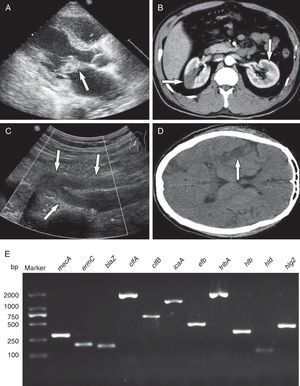
During the next two days, echocardiography revealed large vegetation on the anterior mitral valve leaflet (3cm×3cm) with moderate mitral valve regurgitation (Fig. 1A) Spiral computed tomography (CT) showed renal and splenic infarction (Fig. 1B). CT scans of the brain demonstrated multiple low-density bilateral lesions of the temporal lobes, right parietal lobe and occipital lobe, suggestive of cerebral embolism. Preliminary blood cultures grew S. aureus susceptible to ciprofloxacin, rifampicin, linezolid, vancomycin, tetracycline, sulfamethoxazole, levofloxacin and fosfomycin but resistant to penicillin, oxacillin, clindamycin, cefazolin, cefoxitin, cefuroxime, and erythromycin, as determined on the basis of CLSI disc diffusion standards. A diagnosis of acute infective endocarditis with systemic embolism caused by CA-MRSA was thus considered. Because of his impaired renal function and bacterial susceptibility profile, the patient was treated with intravenous linezolid (600mg q12h) and fosfomycin (8.0g q12h).
Infective endocarditis complicating systemic embolism (the arrows indicate areas of interest) and detection of key genes. (A) Color Doppler echocardiography showing the vegetation attached to the anterior mitral valve leaflet. (B) Spiral computed tomography showing renal infarction. (C) Echocardiographic examination showing embolization of the right common iliac artery and right internal and external iliac arteries. (D) Computed tomography of the brain showing multiple low-density lesions. (E). Detection of antimicrobial genes, virulence-related genes and adhesion genes by PCR amplification. Lane 1, molecular weight marker; lane 2, mecA; lane 3, ermC; lane 4, blaZ; lane 5, clfA; lane 6, clfB; lane 7, icaA; lane 8, efb; lane 9, fnbA; lane 10, hlb; lane 11, hld; lane 12, hlg2.
On day 10, his clinical status worsened with episodes of tachypnea, pink frothy sputum and oxygen saturation (SpO2) decreased rapidly to 83% with ventilator support. Furthermore, renal function deteriorated, oliguria and right lower extremity tissue necrosis appeared. Embolization of the right common iliac artery and right internal and external iliac arteries was seen on echocardiography (Fig. 1C). He subsequently developed a coma with a Glasgow Coma Scale (GCS) score of 6. His clinical condition deteriorated such that he was transferred to the ICU, and replacement of the mitral valve was accomplished with a 29-mm Carbomedics mechanical valve. Considering the presence of coma and fever (39.8°C) postoperatively, brain CT was obtained and showed multiple low-density lesions in temporal lobes, right parietal lobe, and occipital lobe, suggestive of cerebral embolism (Fig. 1D). Levofloxacin (0.75g qd) was added to his antibiotic regimen. After 10 days of intravenous antibiotics, the patient regained consciousness (GCS score of 9) and made a good clinical recovery. On day 35, he was transferred to a secondary hospital, and linezolid therapy was continued for eight weeks. He recovered uneventfully and was well at the last follow-up in November 2013.
Microbiological investigationsThe isolate recovered from the vegetation was first identified by the VITEK 2 system and then identified with MicroFlex LT instrument (Bruker Daltonics). Matrix-assisted laser desorption/ionization time of flight mass spectrometry (MALDI-TOF-MS) based fingerprint analysis of extracted proteins yielded a pattern similar to that of confirmed S. aureus isolates, Flexcontrol 3.0 software and Biotyper 2.0 database (Bruker Daltonics) identified the isolate as S. aureus with a maximum score value of 2.166 (data not shown). In addition to MALDI-TOF-MS analysis, 16S rRNA sequencing was performed in order to identify the origin of the bacteria in the vegetation. The sequence of the PCR product was compared with sequences of closely related species in GenBank by using BLAST. Sequencing of the 16S rRNA gene of the isolates showed that there was 100% identity with the 16S rRNA gene sequence of the isolate of S. aureus (GenBank accession no. JN102565), confirming that the isolate was S. aureus.
To further investigate the genetic basis of the strain, multilocus sequence typing (MLST), a method that uses seven housekeeping genes (arcC, aroE, glpF, gmk, pta, tpi and yqiL) for genetic identification, and result was assigned by comparison with the S. aureus MLST database (http://www.mlst.net/). The staphylococcal chromosomal cassette (SCC) mec type (I–V) was also determined. The spa type was analyzed by sequencing of the PCR product of the spa gene, and the spa type was assigned using an online spa database (http://www.spaserver.ridom.de/). Detection of the accessory gene regulator (agr) allele group was according to PCR and sequencing. Likewise, the antimicrobial drug resistance genes (mecA, msrA, msrB, ermA, ermB, ermC and blaZ) were determined. The presence of gene encoding PVL (lukF/lukS) and other virulence related genes (sea, seb, sec, sed, see, seg, seh, sei, sej, sem, sen, seo, sek, sel, sep, seq, hla, hlb, hld, hlg, hlg2, eta, etb, lukE, lukM, bsaA and edin,) were investigated by PCR. The presence of adhesion genes (cna, clfA, clfB, fnbA, efb and icaA) were also determined by PCR and sequencing.
The CA-MRSA isolate was typed as sequence type (ST) 630 SCCmecV with spa-type t4549, agrI/IV and was PVL-negative. We confirmed the presence of mecA, ermC and blaZ genes by PCR and sequencing. The genome of the MRSA isolate encoded three hemolysin genes (hlb, hld and hlg2) and five adhesion genes (clfA, clfB, fnbA, efb and icaA) (Fig. 1E).
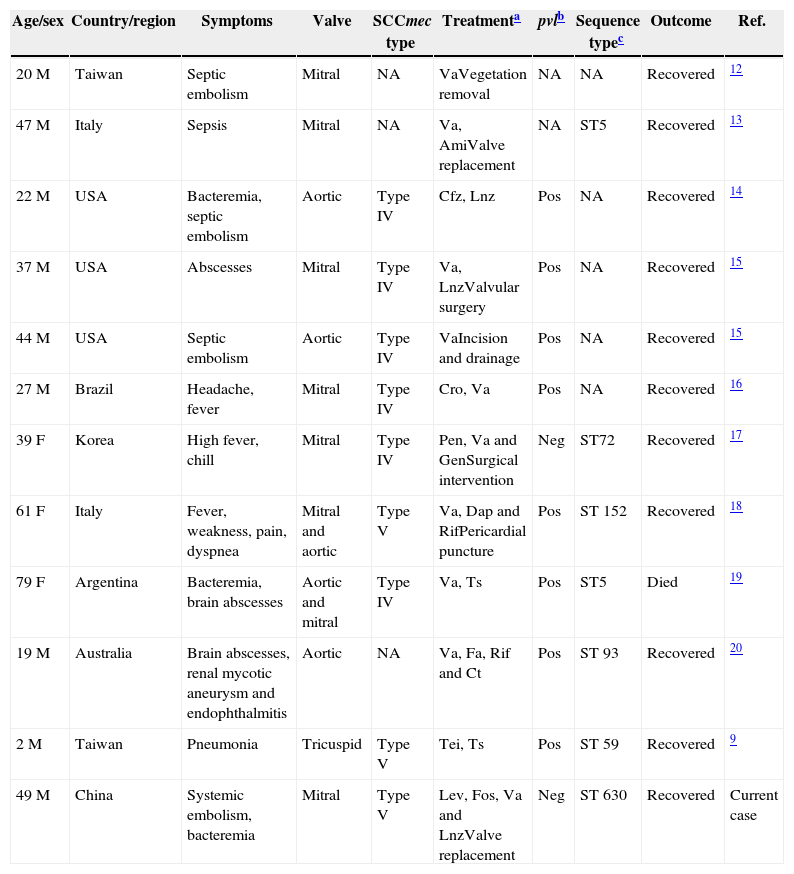
DiscussionIE is a rare entity of CA-MRSA presentation, especially in non-intravenous drug users (IVDU), and CA-MRSA endocarditis cases are mostly restricted to IVDUs, especially among HIV-infected patients with the USA300 strain. To date, 11 previously reported cases of infective endocarditis caused by CA-MRSA, excluding cases in IDVU patients were identified. The clinical features of these cases (including the one described here) are shown in Table 1. Most reported CA-MRSA IE cases were PVL-positive in the list; only two cases were PVL-negative, although one case was untyped. Of the 11 cases collected here, six strains were SCCmec type IV, three strains were SCCmec type V and two untyped strains. These results are consistent with the previous findings. CA-MRSA infections have mostly been epidemiologically linked with strains harboring the SCCmec type IV/V and PVL.4 On the other hand, most CA-MRSA clones are susceptible to many non-β-lactam drugs, and the patient in this case was treated with linezolid, vancomycin, levofloxacin and fosfomycin according to the guidelines. A comprehensive study of CA-MRSA in Chinese children shows that most of the CA-MRSA strains were PVL-negative, and ST59-MRSA-IVa with t437 was the most common clone.5 At pediatric hospitals in five Chinese cities in 2005–2006, of the 73 MRSA clinical isolates, the detection rate of the PVL gene was 30% (22/73), and these included the ST1, ST910, ST88, ST59, and ST338 genetic backgrounds.6 However, the prevalence of CA-MRSA in Beijing was rare among adult SSTI patients.7 In addition, the epidemiological study of CA-MRSA among patients with SSTIs in Hong Kong revealed that the isolates of CA-MRSA strains primarily belonged to the ST30-HKU100 clone and ST59-HKU200 clone.8 In contrast, a previous healthy preschool child was developed IE and pneumonia with pleural effusion by the endemic CA-MRSA clone ST59 in Taiwan.9
Summary of reported cases of CA-MRSA infective endocarditis among patients with no risk factors.
| Age/sex | Country/region | Symptoms | Valve | SCCmec type | Treatmenta | pvlb | Sequence typec | Outcome | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| 20 M | Taiwan | Septic embolism | Mitral | NA | VaVegetation removal | NA | NA | Recovered | 12 |
| 47 M | Italy | Sepsis | Mitral | NA | Va, AmiValve replacement | NA | ST5 | Recovered | 13 |
| 22 M | USA | Bacteremia, septic embolism | Aortic | Type IV | Cfz, Lnz | Pos | NA | Recovered | 14 |
| 37 M | USA | Abscesses | Mitral | Type IV | Va, LnzValvular surgery | Pos | NA | Recovered | 15 |
| 44 M | USA | Septic embolism | Aortic | Type IV | VaIncision and drainage | Pos | NA | Recovered | 15 |
| 27 M | Brazil | Headache, fever | Mitral | Type IV | Cro, Va | Pos | NA | Recovered | 16 |
| 39 F | Korea | High fever, chill | Mitral | Type IV | Pen, Va and GenSurgical intervention | Neg | ST72 | Recovered | 17 |
| 61 F | Italy | Fever, weakness, pain, dyspnea | Mitral and aortic | Type V | Va, Dap and RifPericardial puncture | Pos | ST 152 | Recovered | 18 |
| 79 F | Argentina | Bacteremia, brain abscesses | Aortic and mitral | Type IV | Va, Ts | Pos | ST5 | Died | 19 |
| 19 M | Australia | Brain abscesses, renal mycotic aneurysm and endophthalmitis | Aortic | NA | Va, Fa, Rif and Ct | Pos | ST 93 | Recovered | 20 |
| 2 M | Taiwan | Pneumonia | Tricuspid | Type V | Tei, Ts | Pos | ST 59 | Recovered | 9 |
| 49 M | China | Systemic embolism, bacteremia | Mitral | Type V | Lev, Fos, Va and LnzValve replacement | Neg | ST 630 | Recovered | Current case |
F, female; M, male.
In this case, we report the first infective endocarditis due to novel CA-MRSA ST630 in mainland China. ST630 clone was initially isolated among methicillin susceptible S. aureus (MSSA) as described in S. aureus MLST database and has not been reported in association with human infection. Using MLST and eBURST, the ST 630 MRSA lineage can be grouped as clonal complex 8 (CC8), a large complex of related MSSA and MRSA genotype. ST630 clone is a variant of ST8 clone with the allele at the arcC locus, and ST8 is described as the putative ancestral genotype of another subgroup within CC8. Frequent conversion of MSSA to MRSA by horizontal transfer of SCCmec has been described.10 It is likely that ST630 MRSA originated from ST630 MSSA and ST630 MRSA independently developed by multiple evolutionary pathways. To our knowledge, our case was unique in being the first documented destructive invasive infection case caused by ST630 SCCmecV.
The pathogenetic mechanisms of CA-MRSA infection are not fully understood. However, adhesins have been identified as critical mediators implicated in the induction of experimental S. aureus endocarditis in rats.11 The isolate of this case encodes five adhesion genes might partly contribute to its pathogenesis. In the present high-risk patient, prompt antimicrobial treatment and early surgery were pivotal for avoiding a fatal result. Despite being about only one case, this report highlights the geographical spread and emergence of life-threatening CA-MRSA infection within China.
Conflicts of interestThe authors declare no conflicts of interest.
This work was supported by the National Natural Science Foundation of China (Grant Nos. 81361138021 and 81301461) and the Scientific Research Foundation of Zhejiang Provincial Health Bureau (Grant No. 2012KYB083).