Kocuria rosea belongs to genus Kocuria (Micrococcaceae family, suborder Micrococcineae, order Actinomycetales) that includes about 11 species of bacteria. Usually, Kocuria sp are commensal organisms that colonize oropharynx, skin and mucous membrane; Kocuria sp infections have been described in the last decade commonly affecting immunocompromised patients, using intravenous catheter or peritoneal dialysis. These patients had mainly bacteremia/recurrent sepsis. We hereby describe the case of a 10-year-old girl, immunocompetent, who had endocarditis/sepsis by K. rosea which was identified in five different blood cultures by Vitek 2 ID-GPC card (BioMérieux, France). Negative HIV serology, blood count within normal range of leukocytes/neutrophils and lymphocytes, normal fractions of the complement, normal level of immunoglobulins for the age; lymphocyte immunophenotyping was also within the expected values. Thymus image was normal at chest MRI. No catheters were required. Identification of K. rosea was essential to this case, allowing the differentiation of coagulase-negative staphylococci and use of an effective antibiotic treatment. Careful laboratory analysis of Gram-positive blood-born infections may reveal more cases of Kocuria sp infections in immunocompetent patients, which may collaborate for a better understanding, prevention and early treatment of these infections in pediatrics.
Kocuria rosea belongs to genus Kocuria (Micrococcaceae family, suborder Micrococcineae, order Actinomycetales) that includes about 11 species of bacteria, characteristically gram positive and aerobic (although some species like Kocuria kristinae, Kocuria marina and Kocuria rhizophila may proliferate in anaerobic conditions).1
Usually, Kocuria sp are commensal organisms that colonize oropharynx, skin, and mucous membrane; Kocuria sp infections have been described in the last decade commonly affecting immunocompromised patients, using intravenous catheter or peritoneal dialysis. These patients had mainly bacteremia/recurrent sepsis.1–5 It is noticeable, however, that the immunocompromise was not mandatory in all reported cases.3
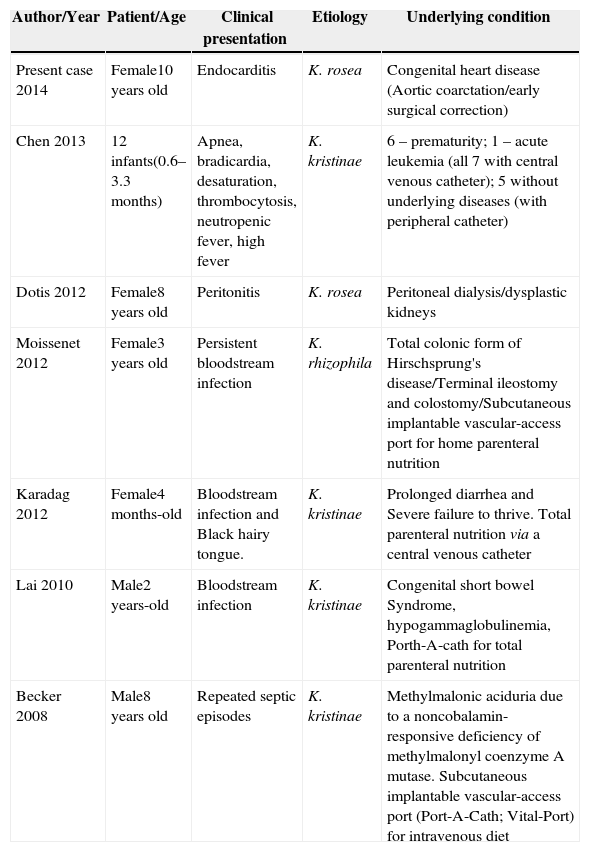
Most pediatric cases were caused by K. kristinae; K. rosea was only described in one child until now, with peritonitis2–7 (Table 1).
Reported cases of Kocuria sp infections in pediatric patients.
| Author/Year | Patient/Age | Clinical presentation | Etiology | Underlying condition |
|---|---|---|---|---|
| Present case 2014 | Female10 years old | Endocarditis | K. rosea | Congenital heart disease (Aortic coarctation/early surgical correction) |
| Chen 2013 | 12 infants(0.6–3.3 months) | Apnea, bradicardia, desaturation, thrombocytosis, neutropenic fever, high fever | K. kristinae | 6 – prematurity; 1 – acute leukemia (all 7 with central venous catheter); 5 without underlying diseases (with peripheral catheter) |
| Dotis 2012 | Female8 years old | Peritonitis | K. rosea | Peritoneal dialysis/dysplastic kidneys |
| Moissenet 2012 | Female3 years old | Persistent bloodstream infection | K. rhizophila | Total colonic form of Hirschsprung's disease/Terminal ileostomy and colostomy/Subcutaneous implantable vascular-access port for home parenteral nutrition |
| Karadag 2012 | Female4 months-old | Bloodstream infection and Black hairy tongue. | K. kristinae | Prolonged diarrhea and Severe failure to thrive. Total parenteral nutrition via a central venous catheter |
| Lai 2010 | Male2 years-old | Bloodstream infection | K. kristinae | Congenital short bowel Syndrome, hypogammaglobulinemia, Porth-A-cath for total parenteral nutrition |
| Becker 2008 | Male8 years old | Repeated septic episodes | K. kristinae | Methylmalonic aciduria due to a noncobalamin-responsive deficiency of methylmalonyl coenzyme A mutase. Subcutaneous implantable vascular-access port (Port-A-Cath; Vital-Port) for intravenous diet |
Laboratory identification of Kocuria sp by biochemistry methods is difficult due to similarity with other pathogens, especially coagulase-negative staphylococci, which delays the proper treatment.1,3
We herein describe a case of a 10-year-old girl who was diagnosed with aortic coarctation, which was surgically corrected at the age of 11 days. Since then she has had compensated congestive heart failure using propranolol and furosemide. This girl had appropriate weight and height for her age and no other co-morbidity during her life. At the age of 10 years she began having daily fever (without identified cause) and splenomegaly. Thirty days after the first fever episode, she had clinical and radiological diagnosis of pneumonia, at first treated with oral clarithromycin. After one week of treatment, the patient had again fever associated with headache, heart failure, and signs of sepsis; complementary imaging evaluation showed subarachnoid hemorrhage and bacterial endocarditis (vegetation in the mitral valve). During this period, five blood cultures, in three different days, were positive for K. rosea. The patient responded well to intravenous amoxicillin and clavulanate and support measures (oxygen by mask, diuretics); central intravenous catheter or other invasive procedures were not required.
As Kocuria sp infection is classically related to immunodeficiency, a specific investigation was carried out that negative for HIV infection, leukocytes/neutrophils and lymphocytes within normal range, normal fractions of the complement, normal levels of immunoglobulins for the age; lymphocyte immunophenotyping was also within the expected values. The thymus looked normal at chest MRI.
Kocuria sp laboratory identification was performed in a three-phase blood sample system (Probac); five samples, on three different days (day 0, 3 and 28), were positive for Gram-positive cocci in tetrads; the colony grew under aerobic conditions at 37°C; replication took place in 5% sheep blood medium (BioMérieux, France). Kocuria sp identification was performed by Vitek 2 ID-GPC card (BioMérieux, France). Analysis of the genome through molecular methods is desirable, but due to economic and technical limitations its use was not possible in our service.
Infective endocarditis (IE) is a disease with high mortality rate, despite medical advances. IE is uncommon in children under 17-year-old and most cases are associated with structural heart defects. A recent Canadian study involving 136 children with IE showed that cyanotic congenital cardiopathy and surgical correction before six months old were major risk factors.8 In the case shown here, both situations were present. The most common etiological agents for IE are Streptococcus viridans, Staphylococcus aureus, coagulase-negative Staphylococcus, and Streptococcus pneumoniae. Enterococcus and other Gram-negative are rare. The genus Kocuria is considered an atypical cause of endocarditis1; one case of IE by K. rosea was described in a 35-year-old woman, but no cases have been described in children.9 Regarding antibiotic susceptibility, Kocuria sp is sensitive to a variety of drugs (amoxicillin, cephalosporin, aminoglycoside, vancomycin, clindamycin); variable sensitivity to quinolones and sulfa.3,9 Amoxicillin-clavulanate has been proposed as the initial antibiotic treatment,1 as done in this case. Potentially contaminated catheters, if present, must be removed.
Many aspects of Kocuria sp infections are not yet entirely understood; besides human and other mammals, these bacteria may be found in drinking water sources, different sediments, seed and fermented food, being notable for its tropism for plastics. Kocuria sp usually form a biofilm, frequently in association with other bacteria.2 A recently identified K. rosea strain (BS1) is capable of producing an exopolysaccharide (called Kocuran), that has, in vitro, antioxidant and immunosuppressive properties – in human polymorphonuclear cultures stimulated with PHA, Kocuran inhibit the proliferation of these cells and also inhibit complement mediated hemolysis.10
There are few sporadic reports of Kocuria sp infections (especially by K. rosea); in the present case, despite IE risk factors, the lab screening for primary immunodeficiency was negative and there was no prolonged use of any kind of catheters. Genomic methods, as 16S RNA gene sequence, are desirable for correct identification of coagulase-negative staphylococci which presents a large phenotypic variation; this kind of approach is equally useful to confirm Kocuria species.11 However, despite not having used genomic methods and some restriction about Vitek 2 ID GPC card,11,12 identification of K. rosea was essential in this case, and subsequent use of effective antibiotic treatment. Careful laboratory analysis of Gram-positive blood infection may reveal more cases of Kocuria sp infections in immunocompetent patients, which may contribute for better understanding, prevention, and early treatment of these infections in pediatrics.
Conflicts of interestThe authors declare no conflicts of interest.