The etiology of pulmonary infections in HIV patients is determined by several variables including geographic region and availability of antiretroviral therapy.
Materials and methodsA cross-sectional prospective study was conducted from 2012 to 2016 to evaluate the occurrence of pulmonary fungal infection in HIV-patients hospitalized due to pulmonary infections. Patients’ serums were tested for (1–3)-β-D-Glugan, galactomannan, and lactate dehydrogenase. The association among the variables was analyzed by univariate and multivariate regression analysis.
Results60 patients were included in the study. The patients were classified in three groups: Pneumocystis jirovecii pneumonia (19 patients), community-acquired pneumonia (18 patients), and other infections (23 patients). The overall mortality was 13.3%. The time since diagnosis of HIV infection was shorter in the pneumocystosis group (4.94 years; p=0.001) than for the other two groups of patients. The multivariate analysis showed that higher (1-3)-β-D-Glucan level (mean: 241pg/mL) and lactate dehydrogenase (mean: 762U/L) were associated with the diagnosis of pneumocystosis. Pneumocystosis was the aids-defining illness in 11 out of 16 newly diagnosed HIV-infected patients.
ConclusionIn the era of antiretroviral therapy, PJP was still the most prevalent pulmonary infection and (1-3)-β-D-Glucan and lactate dehydrogenase may be suitable markers to help diagnosing pneumocystosis in our HIV population.
The epidemiology of pulmonary infections varies according to geographical regions and to whether the infection occurred before or after active antiretroviral therapy (ART) availability. In some low-income countries, tuberculosis and pneumocystosis are still the most frequent pulmonary complications in HIV/AIDS patients.1 Conversely, in higher resources settings, the frequency of pulmonary diseases associated with AIDS has decreased due to the benefits of the ART.2–4 Hospitalization due to chronic obstructive respiratory disease, lung cancer, and bacterial pneumonia has become more frequent than pulmonary infections due to Pneumocystis jirovecii, tuberculosis, and cytomegalovirus.5,6 However, lower respiratory tract infections (LRTI) are still 25-fold more common in the HIV population compared to the general community causing an estimate number of 20–25 episodes per 100 hospitalizations worldwide.6,7
Fungal infections in HIV-infected patients are neglected diseases, predominantly in countries with limited resources, and represent a significant cause of pulmonary infections. The burden of HIV-related mycosis worldwide is estimated to account, per year, for more than 950,000 cases of cryptococcosis, 400,000 cases of PJP, and 300,000 of disseminated histoplasmosis.8
The majority of the diagnoses of pulmonary diseases are based on clinical symptoms and X-ray findings resulting in patients being treated empirically. Identification of the etiologic agent is a complex task requiring the combination of microbiologic exams, serologic markers, molecular techniques, and invasive procedures, such as lung biopsy and bronchoalveolar lavage (BAL). New molecular techniques have been studied to improve the diagnosis of pulmonary infiltrates in the HIV-infected population. Loop-mediated isothermal amplification (LAMP) has been evaluated for the detection of Pneumocystis DNA in respiratory specimens and could serve as a diagnostic tool.9 Serum (1-3)-β-D-Glugan (BG) has been a promising non-culture method for the diagnosis of some fungal infections, including, Candida spp., Fusarium, and P. jirovecii. Recent data have shown that serum BG could be correlated with the diagnosis of PJP10–12 with a sensitivity range of 90–100% and specificity of 65–100%.13–15
In low/middle income countries there is an urgent need for prospective studies to clarify the infectious causes of pulmonary diseases that lead to hospitalizations in AIDS patients. In this scenario, the proper identification of the causative agents will indicate a better therapeutic approach and improve the understanding of the epidemiology of pulmonary affections responsible for hospitalizations in our country.
The aim of this study was to evaluate the occurrence of pulmonary fungal infections and the role of serum markers in HIV-infected patients hospitalized with acute respiratory symptoms, in a tertiary care referral hospital in Campinas, Sao Paulo, Brazil.
Materials and methodsStudy populationWe conducted a prospective cross-sectional study, from 2012 to 2016, at the Hospital das Clinicas of the University of Campinas (UNICAMP), Sao Paulo, Brazil, which is the reference hospital for more than six million inhabitants.
The Ethical Committee approved this study (No. 8876/2012) and all patients signed the informed consent form. The inclusion criteria included HIV infection, age over 18 years old, and hospitalization due to symptoms of lower respiratory tract infection. Exclusion criteria included pregnant women and patients with nosocomial pulmonary infections. At the time of hospitalization, a physiotherapist (AIPM) performed a pulmonary examination in all patients and collected sputum and oral lavage for the molecular diagnosis of P. jirovecii. A radiologist assessed all chest radiographies and tomographies. For the assessment of the pneumonia severity, the patients were classified using CURB-65 score.16 At the day of admission, the following data were abstracted from the patients’ records: age, gender, duration of HIV infection (in years), duration of hospitalization (in days), outcome, previous opportunistic infections, hemoglobin level, hematocrit, total leukocytes, serum creatinine, serum urea, CD4 T cell count, and HIV viral load in the last two months. In addition, serum cryptococcal antigen, serology for paracoccidioidomycosis, blood culture for bacteria and fungi, culture for mycobacteria and fungi in sputum, serum LDH, BG and GM, LAMP of respiratory specimens (sputum, BAL) and oral lavage (OL) for P. jirovecii were also tested.
The clinical diagnosis of pneumonia due to P. jirovecii was established according to WHO clinical criteria of case definition for HIV-related opportunistic diseases,17 and a chest X-ray showing bilateral interstitial infiltrates or a chest tomography showing alterations compatible with PJP (bilateral patchy ground grass opacity with a central perihilar predominance). A definitive diagnosis was based on the identification of P. jirovecii in cytology or immunofluorescent microscopy of induced sputum or BAL or histology of lung tissue.
Community-acquired pneumonia (CAP) was defined as the presence of cough together with one or more of the symptoms: chest pain, dyspnea, presence of new pulmonary infiltrate on chest radiography.18 Criteria for diagnosis of CAP and LRTI due to bacteria, fungi other than P. jirovecii, or parasites were based on the identification of the etiologic agent in cultures of blood and respiratory secretions.
BG and GM in serum and in BAL specimenSerum and BAL samples were tested for GM and BG. Concentrations of GM were determined by using the Platelia Aspergillus Ag assay (Bio-Rad, Marnes-la-Coquette, France), according to the manufacturer's instructions. An OD≥0.5 was considered as a positive result. BG levels were determined by the Fungitell® assay (Associates of Cape Cod, Inc., Cape Cod, MA, USA), according to the manufacturer's recommendations. BG levels ≥80pg/mL were considered as positive results.
LAMP for P. jiroveciiSputum, oral lavage, and BAL samples were tested for P. jirovecii by LAMP. DNA extraction procedures strictly followed the instructions defined by the manufacturer (Eiken Chemical Co., Tokyo, Japan) using the oligonucleotide primers specific for P. jirovecii.19
Statistical analysisCategorical variables were analyzed using chi-square, Fisher, and Mid-P exact tests, and for continuous variables t-test, ANOVA and Mann–Whitney/Wilcoxon test were used. A p-value <0.05 was considered statistically significant. The multivariate stepwise logistic regression analysis was performed to determine the factors independently associated with fungal infection. The following programs were used: Epi info v.7 (Centers for Disease Control and Prevention, Atlanta, USA), Open Source Epidemiologic Statistics for Public Health, version 3.03a updated 2015/05/04,20 and SAS System for Windows (version 9.1.3, SAS Institute, Cary, North Carolina).
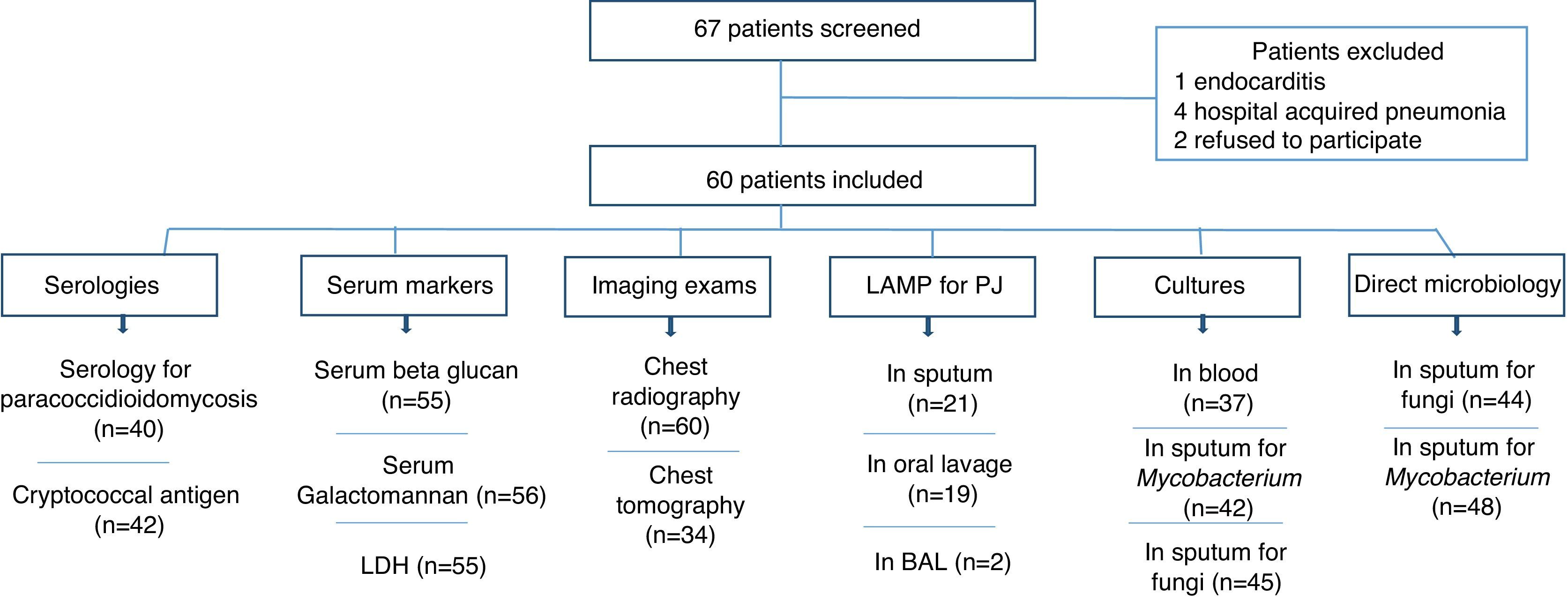
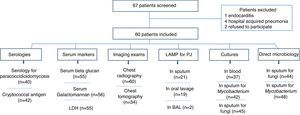
ResultsA total of 67 HIV-infected patients were included. Fig. 1 shows the distribution of patients according to results of the exams performed (imaging, biomarkers, LAMP, serologies, and microbiologic exams).
The patients were classified into three groups: (a) PJP (19 cases): included patients with PJP and patients with diagnosis of PJP plus another pulmonary infection/disease; (b) patients with CAP (18 cases); and (c) other diagnosis (23 cases of lower respiratory infection: nine cases of tuberculosis, five cases of histoplasmosis, two cases of nocardiosis, one case of cryptococcosis, one case of disseminated strongyloidiasis, one case of pulmonary embolism, and three undiagnosed cases). PJP and CAP were the most frequent diagnosis representing 31.6% and 30%, respectively. Tuberculosis was diagnosed in five (8.3%) patients. Seven patients with CAP had positive blood cultures for bacteria (one Acinetobacter baumanii, one Kokuria micrococcus, and five Streptococcus pneumoniae). Serology for paracoccidioidomycosis and detection of cryptococcal antigen in serum were assessed in 40 and 42 patients, respectively. Only the patient, with pulmonary cryptococcosis, had a positive cryptococcal antigen. All serologies for paracoccidioidomycosis turned out negative. The two patients with histoplasmosis had cultures of skin biopsies positive for Histoplasma.
Direct sputum smear microscopy and sputum culture for mycobacteria were examined in 48 and 42 patients respectively, and for fungi in 44 and 45 patients, respectively. Direct sputum smear was positive for fungi in seven cases (five yeasts and filamentous fungi, one Strongyloides stercoralis and one non-pathogenic yeast) and for Mycobacterium in four patients. Sputum culture for fungi was positive in 24 cases (18 non-pathogenic yeast, three Candida albicans, one Actinomyces spp., one Cryptococcus neoformans and one Histoplasma capsulatum) and for Mycobacterium in four cases (two Mycobacterium tuberculosis and two Mycobacterium avium).
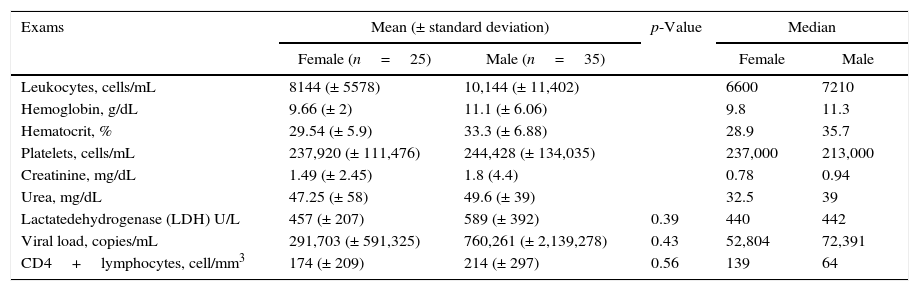
The mean values of hemoglobin and hematocrit were below the reference values (Table 1). Forty-one percent (25 patients) were female. The median time since HIV diagnosis was 10 years; however, 22.8% of the patients had less than one year. HIV infection was first diagnosed at hospitalization in 16 (26.6%) patients; PJP was the AIDS-defining illness in 11 patients; the mean age of patients was 43.4 years (female: 43.8; male: 43.1); 42 (70%) patients had less than 250 cell/mm3 CD4 cell count and only 14 (23.3%) patients had HIV viral load <50 copies/mL. The means of HIV viral load and CD4 T cell count for males (760,261 copies/mL; 214 cells/mm3) and females (291,703 copies/mL; 174 cells/mm3) were not significantly different (p=0.43 and p=0.56, respectively) (Table 1).
Laboratory data of 60 HIV-infected patients hospitalized with pulmonary infections.
| Exams | Mean (± standard deviation) | p-Value | Median | ||
|---|---|---|---|---|---|
| Female (n=25) | Male (n=35) | Female | Male | ||
| Leukocytes, cells/mL | 8144 (± 5578) | 10,144 (± 11,402) | 6600 | 7210 | |
| Hemoglobin, g/dL | 9.66 (± 2) | 11.1 (± 6.06) | 9.8 | 11.3 | |
| Hematocrit, % | 29.54 (± 5.9) | 33.3 (± 6.88) | 28.9 | 35.7 | |
| Platelets, cells/mL | 237,920 (± 111,476) | 244,428 (± 134,035) | 237,000 | 213,000 | |
| Creatinine, mg/dL | 1.49 (± 2.45) | 1.8 (4.4) | 0.78 | 0.94 | |
| Urea, mg/dL | 47.25 (± 58) | 49.6 (± 39) | 32.5 | 39 | |
| Lactatedehydrogenase (LDH) U/L | 457 (± 207) | 589 (± 392) | 0.39 | 440 | 442 |
| Viral load, copies/mL | 291,703 (± 591,325) | 760,261 (± 2,139,278) | 0.43 | 52,804 | 72,391 |
| CD4+lymphocytes, cell/mm3 | 174 (± 209) | 214 (± 297) | 0.56 | 139 | 64 |
Reference values. Creatinine: adults – men<1.20mg/dL; women<0.90mg/dL; urea: adult≤65 years: 16.6–48.5mg/dL and adult>65 years: <71mg/dL; LDH: adult≤65 years: 240–480U/L; leukocytes: adults – 4000–10,000 cell/mL; hemoglobin: adults – men 14–18g/dL, women 12–16g/dL; hematocrit: adults – men 41–52%, women 36–46%; platelets: adults – 150,000–400,000 cells/mL.
Mean time of hospitalization was 19 days. The length-of-stay in hospital was not associated with outcome (p=0.21; death: 27.9 days; survival: 16.3 days). Nine (15%) patients died during hospitalization (4 PJP, 3 CAP, 1 tuberculosis, and 1 disseminated strongyloidiasis). The following variables were not associated with the outcome: age (p=0.40), gender (p=0.46), CD4 T cell count (p=0.15), HIV viral load (0.37), LDH (0.09), BG (0.08), and time since HIV diagnosis (p=0.45).
GM was analyzed in serum of 56 patients and in BAL of two patients. One patient had serum and BAL positives (PJP) and one patient had positive serum sample (CAP). All the other GM tests were negative.
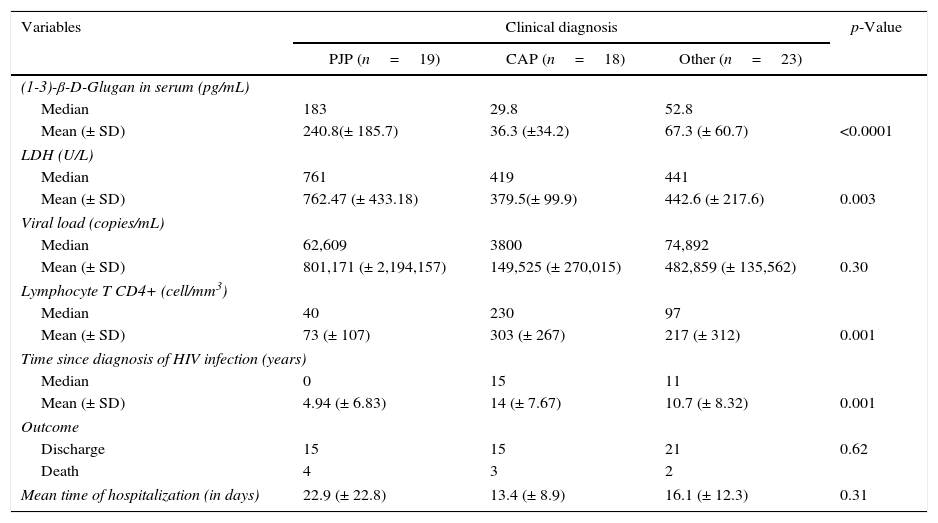
Results of univariate analysis of the three groups of patients are summarized in Table 2. The mean values of BG in the group of patients clinically diagnosed as P. jirovecci (19 patients; 240.8±185.7pg) was significantly higher than in patients with CAP (18 patients; 36.3±34.2pg; p<0.0001) and in those diagnosed with other infections (23 patients; 67.3±60.7pg; p<0.0001). Eight patients not diagnosed with PJP had BG>80pg/mL (histoplasmosis: 1; strongyloidiasis: 1; tuberculosis: 1; cryptococcosis: 1; CAP: 2; undiagnosed: 2). Considering the BG cut-off of >80pg/mL, the sensitivity and specificity of BG in the PJP patients were 0.90 and 0.80, respectively. Two patients with PJP had BG<80pg/mL. LDH was also significantly higher in the group of patients with P. jirovecii (mean 762.47±433.18U/L) than in patients with CAP (379.5±99.9U/L), and other diagnosis (442.6±217.6U/L) (p=0.003). Patients with PJP had lower T CD4+lymphocyte count (p=0.001) and duration of time since HIV diagnosis (p=0.001). However, higher BG and LDH were the only independent variables associated with the diagnosis of PJP in relation to the diagnosis of CAP and other infections (Table 3).
Univariate analysis of the (1-3)-β-D-Glugan, LDH, HIV viral load, CD4 T cell count, duration of HIV infection, and outcome of the three groups of patients included in the study with pulmonary infections.
| Variables | Clinical diagnosis | p-Value | ||
|---|---|---|---|---|
| PJP (n=19) | CAP (n=18) | Other (n=23) | ||
| (1-3)-β-D-Glugan in serum (pg/mL) | ||||
| Median | 183 | 29.8 | 52.8 | |
| Mean (± SD) | 240.8(± 185.7) | 36.3 (±34.2) | 67.3 (± 60.7) | <0.0001 |
| LDH (U/L) | ||||
| Median | 761 | 419 | 441 | |
| Mean (± SD) | 762.47 (± 433.18) | 379.5(± 99.9) | 442.6 (± 217.6) | 0.003 |
| Viral load (copies/mL) | ||||
| Median | 62,609 | 3800 | 74,892 | |
| Mean (± SD) | 801,171 (± 2,194,157) | 149,525 (± 270,015) | 482,859 (± 135,562) | 0.30 |
| Lymphocyte T CD4+ (cell/mm3) | ||||
| Median | 40 | 230 | 97 | |
| Mean (± SD) | 73 (± 107) | 303 (± 267) | 217 (± 312) | 0.001 |
| Time since diagnosis of HIV infection (years) | ||||
| Median | 0 | 15 | 11 | |
| Mean (± SD) | 4.94 (± 6.83) | 14 (± 7.67) | 10.7 (± 8.32) | 0.001 |
| Outcome | ||||
| Discharge | 15 | 15 | 21 | 0.62 |
| Death | 4 | 3 | 2 | |
| Mean time of hospitalization (in days) | 22.9 (± 22.8) | 13.4 (± 8.9) | 16.1 (± 12.3) | 0.31 |
PJP, Pneumocystis jirovecii pneumonia. PJP group included: P. jirovecii+community acquired pneumonia: 4 patients; P. jirovecii+Mycobacterium non-tuberculosis: 2 patients. CAP: community acquired pneumonia. Other: lower respiratory infection: 9 patients; tuberculosis: 5 patients; histoplasmosis: 2 patients; cryptococcosis: 1 patient; disseminated strongyloidiasis: 1 patient; nocardiosis: 1 patient; pulmonary embolism: 1 patient and undiagnosed: 3 patients.
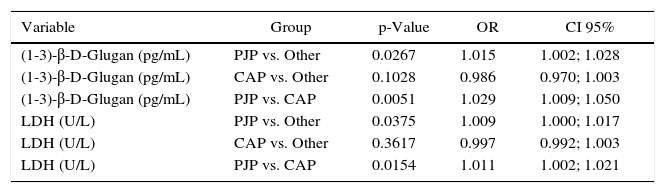
Multivariate logistic regression analysisa of variables independently associated with P. jirovecii pneumonia, community-acquired pneumonia, and other pulmonary diseases.
| Variable | Group | p-Value | OR | CI 95% |
|---|---|---|---|---|
| (1-3)-β-D-Glugan (pg/mL) | PJP vs. Other | 0.0267 | 1.015 | 1.002; 1.028 |
| (1-3)-β-D-Glugan (pg/mL) | CAP vs. Other | 0.1028 | 0.986 | 0.970; 1.003 |
| (1-3)-β-D-Glugan (pg/mL) | PJP vs. CAP | 0.0051 | 1.029 | 1.009; 1.050 |
| LDH (U/L) | PJP vs. Other | 0.0375 | 1.009 | 1.000; 1.017 |
| LDH (U/L) | CAP vs. Other | 0.3617 | 0.997 | 0.992; 1.003 |
| LDH (U/L) | PJP vs. CAP | 0.0154 | 1.011 | 1.002; 1.021 |
PJP, P. jirovecii pneumonia; CAP, community-acquired pneumonia; Other, other pulmonary diseases; LDH, lactate dehydrogenase; OR, Odds Ratio; CI, confidence interval.
Eleven (68.7%) of the 16 patients who were first diagnosed as HIV during hospitalization had PJP (nine patients) or PJP associated with Mycobacterium non-tuberculosis (two patients) and of the other five, two patients had histoplasmosis, one cryptococcosis, one tuberculosis, and one patient had LRTI.
Tuberculosis (17 cases), Pneumocystosis (16 cases), Herpes zoster (13 cases), cerebral toxoplasmosis (10 cases) and hepatitis C (11 cases) were the most prevalent previous opportunistic infections reported in our patients. Six patients included in this study had a previous diagnosis of PJP. Three patients out of the 17 previously diagnosed with tuberculosis had a treatment failure or relapsed.
Thirty-five patients had at least one clinical specimen (BAL: 1, sputum: 14; OL: 13; sputum+OL: 6; sputum+BAL: 1) tested by LAMP. This molecular method detected P. jirovecii DNA product in clinical specimen of five patients (1 BAL; 4 sputum). All patients whose LAMP turned out positive had chest X-ray or CT suggestive of PJP and BG≥341.55pg. LAMP were preformed in 12 patients with PJP and only in five it turned out positive.
DiscussionPulmonary infection is one of the most frequent complication in people living with HIV/AIDS, remaining the leading cause of morbidity and mortality worldwide.7 In our study, pneumocystosis, CAP, tuberculosis, and LRTI were the most frequent causes of hospitalization. Of note, fungal infections represented 36.6% (22 patients) of our cases. Pneumocystosis was the most frequent AIDS-defining opportunistic infection representing 68.7% of newly diagnosed AIDS cases, underscoring the need to improve early detection of HIV infection. Remarkably, tuberculosis was AIDS-defining illness in only one patient.
Systematic reviews have demonstrated that opportunistic infections decreased after the availability of ART, mostly in high-income countries.21 However, there is a lack of information on the epidemiology of opportunistic infections in low- and middle-income countries, including Brazil. Our study demonstrated that opportunistic infections were still prevalent. A study from San Francisco reported that PJP (39.1%) followed by Kaposi sarcoma (20.1%) were the most common AIDS-defining diseases.22 Grinsztejn et al.23 found tuberculosis as the most common AIDS-defining opportunistic infection in a Brazilian cohort compared to esophageal candidiasis among US HIV-infected patients. Another study from Rio de Janeiro, concluded that tuberculosis was the most frequent AIDS-defining opportunistic illness,24 whereas in a recent series of HIV-infected patients from a referral hospital in Brazil, pneumocystosis was still the prevailing opportunistic infection in the HIV group.25 The difference between the rates of opportunistic infections found in our results compared to other studies could be explained by the causes of pulmonary infections we have studied and not all causes of opportunistic infections in AIDS patients. Notably, most international and national studies are from single centers. Although their results contributed to the understanding of the local epidemiology, the conduct of multicenter studies will lead to better prevention and treatment strategies. One limitation of this study was the fact that only 60 AIDS patients met the inclusion criteria during the study period. The time frame of the study, the limited number of beds in the infectious diseases ward, and the long length hospital stay were external factors that contributed in reducing the sample size of our study.
Clinicians search for non-invasive methods, such as serum markers, as an aid to diagnose pulmonary infiltrates, particularly in HIV/AIDS. BG values were considerably higher in patients clinically diagnosed with PJP and might be a good marker for patients with AIDS and pulmonary symptoms. The visualization of the P. jirovecii in pulmonary specimens is difficult, whereas the dosage of serum BG could be a reliable marker in the diagnosis of PJP.10 Considering the BG cut-off value recommended by the manufacturer (80pg/mL), we found a high sensitivity (0.90) and specificity (0.80). In two other studies, that included a higher number of HIV patients than our study, the authors found, using the same cut-off, a sensitivity and specificity of 0.91/0.64 and 0.98/0.39, respectively.10,26 One potential limitation of the use of BG in clinical practice with HIV-infected patients might be the positivity of the test in other invasive mycosis, such as candidiasis, fusariosis, aspergillosis, and histoplasmosis.27,28
Previous studies have found that high levels of LDH were associated with PJP, and that LDH could be a serum marker in the diagnosis of pneumocystosis.10,29,30 Our data showed a significant correlation between high levels of LDH and pneumocystosis (p=0.003) (Table 2). The multivariate analysis showed that higher levels of BG and LDH were independently associated with PJP when compared with the results in patients with CAP and other pulmonary conditions (Table 3). In the multivariate logistic regression analysis described in Table 3 the odds ratios represent the effect of the increment of each “1” unit of BG (pg/mL) or LDL (U/L) levels, in the chance of a patient having PJP, as these variables were continuous variables.31 Based in our results, the combination of more than one serum marker may be helpful in the diagnosis of PJP and to discriminate PJP from other pulmonary diseases.
LAMP is a rapid simple and cost-effective method for diagnosing various pathogens including Pneumocystis.9 LAMP for P. jirovecii was tested in the respiratory secretions of our patients and the results were not encouraging. Most of the clinical samples from our patients were sputum or OL, and perhaps if we had tested BAL, we would have obtained better results. However, in clinical practice, non-invasive diagnostic methods are preferred over invasive procedures, such as BAL or biopsies. In our series of cases, serum BG and LDH provided better aid for the diagnosis of pneumocystosis than LAMP.
In conclusion, our results showed that PJP still has a higher prevalence over other pulmonary conditions in our geographic area, and that we need to implement prompt and accurate diagnostic tests for pulmonary infections. BG and LDH showed to be suitable serum markers in the diagnosis of PJP and should be implemented in routine care to assist our HIV/AIDS population. Moreover, most of our patients were previously enrolled in a HIV/AIDS care center. Although, we have a well-established National program for HIV/AIDS in Brazil, the HIV cascade care is neglecting the early diagnosis, patient follow-up, in addition to insufficient patient adherence to the HIV/AIDS program. This study showed that there is a need for better strategies to achieve early diagnosis of HIV infection and therefore reducing opportunistic infections in the infected patients.
Conflicts of interestThe authors have no conflicts of interest.
This project was supported by grants from the collaborative research project: Science and Technology Research Partnership for Sustainable Development, Japan (SATREPS) and University of Campinas, Brazil, No. 02P-29548-09 and Fundação de Amparo à Pesquisa do Estado de Sao Paulo, FAPESP No. 2012/51158-0. We are grateful to Mr. Roger Timothy Rentfrow for the English proofreading.