Brazil is characterized by a concentrated AIDS epidemic, it has a prevalence of less than 1% in the general population. However, there are higher rates in specific populations, especially in men who have sex with men. The study's aim was to analyze the association between sociodemographic characteristics, sexual practices, sexual behaviors and the HIV infection in a group of men who have sex with men. Secondary data was collected between June 2014 and September 2015 in a research of cross-sectional design in the city of Rio de Janeiro, Brazil. Volunteers answered an online computerized questionnaire and took HIV test. Chi-squared distribution and multiple logistic regression was used. There were 341 participants. Most of them were racially mixed, single, average age of 30.6 years and with a higher education level. The HIV prevalence was 13.9%. Two logistic models were fit (insertive or receptive anal intercourse). Both models showed an association with HIV among those who had a HIV positive sexual partner (Odds Ratio≈2.5) and a high self-perception of acquiring HIV (Model 1: Odds Ratio≈7/Model 2: Odds Ratio≈10). Low condom usage in receptive anal intercourse with casual partners had a direct association with HIV seropositivity, whereas insertive anal intercourse with casual partners with or without condoms were inversely related. The study identified a high prevalence of HIV infections among a group of men who sex with men with a high self-perception risk of acquiring HIV. The findings also showed a relation with sociodemographic and sexual behavior variables.
Despite the introduction of antiretroviral therapy since the 1990s in Brazil,1,2 the Acquired Immune Deficiency Syndrome (AIDS) remains one of the most serious public health problems.3–5 Brazilian studies have shown that a large proportion of individuals diagnosed with Human Immunodeficiency Virus (HIV) still started treatment late.6–8 Szwarcwald et al. showed that the delay between infection and date of first CD4 count was 4.3 years.7
In 2014, it was estimated that 36.9 million people were living with HIV worldwide, 80% concentrated in just 20 countries, Brazil being one of them. Moreover, against the global downward trend in new HIV infections between 2000 and 2014, Brazil recorded an increase in new cases of HIV.9
In Brazil, the HIV incidence rate in 2013 among those aged 15 years and over was estimated in 29.4/100,000 population; higher among men (43.5/100,000) than women (15.9/100,000).7 Another study in men who have sex with men (MSM) showed a high HIV incidence in two Brazilian cities ranging from 1.47% to 0.92% in 2013.10
The prevalence of HIV in the general population of Brazil was 0.4% in 2014. However, in specific subpopulations such rates were higher. Studies conducted between 2008 and 2009 found an HIV prevalence rate of 10.5% among men who had sex with men (MSM).11 Other studies in Brazilian cities also showed HIV prevalence in MSM around 10% or higher.12,13 In the general population the AIDS prevalence remained stable less than 1% over the years characterizing Brazil as a country of a concentrated epidemic. This means that HIV/AIDS is spread mainly in specific populations at higher risk, with prevalence rates higher than 5% in these subpopulations.14–16
The findings of a survey conducted in two Brazilian cities in 2013 showed that most of the new infections were in MSM, at about 40%.10 The increased risk of infection for HIV among MSM has been associated with sexual attitudes and behaviors like sex with casual partners, use of illicit drugs and alcohol during sex, receptive unprotected anal sex, sex role versatility among MSM, and use of the internet to find sexual partners.14,17–21 Given this background, in which MSM has been disproportionately affected by HIV infections, this study aimed to analyze the association between sociodemographic characteristics, sexual practices, and behaviors with HIV/AIDS in a sample of MSM residents in the city of Rio de Janeiro (RJ).
Material and methodsThe study used primary data from a social network analysis research collected between June 2014 and September 2015 entitled “The use of social network analysis to study variables associated with HIV infection among MSM” held in the city of Rio de Janeiro. This study was submitted and approved by the Research Ethics Committee of the National School of Public Health, Fiocruz (ENSP/Fiocruz) and was funded by the Department of Public Health Control, Prevention and Monitoring of sexually transmitted infections, AIDs and Viral Hepatitis (DDAHV/SVS/MS) of the Ministry of Health in collaboration with the United Nations’ Office on Drugs and Crime (UNODC) and the State Health Secretary of Rio de Janeiro.
This was a cross-sectional study conducted in two Civil Society Organizations (OSC), and a Primary Health Unit located in the city of Rio de Janeiro. Inclusion criteria were: sexually active men aged 18 and over, who reported homosexual or bisexual behavior in the last six months, unaware of their HIV status or having the last HIV testing negative. The study used a convenience sampling. Study participants were recruited in two ways: (1) individuals participating in other activities of OSC or the Primary Health Care Unit on the day of testing were invited to participate; (2) active recruitment, in which individuals in MSM venues were invited to learn more about the research getting a card with the location and time of the study.
Data was collected online at the study locations by the use of an adapted structured questionnaire22 in the Survey Gizmo software. The questionnaire was self-administered and the participants filled it out in private offices. Sex was defined as any type of oral, vaginal or anal encounters, with or without ejaculation, involving two or more persons, regardless of the situation in which the sexual contact occurred or the type of relationship with the other person. Regular partners were defined as those with whom they have had sex and defined as an affair, dating, frequent meetings, wedding, or any kind of involvement. Casual partners were considered those who have had sex with no subsequent encounters or without establishing any commitment to the relationship. Anonymous partners were defined as those who have had sex without even knowing the person and not knowing how to find the person again. Protected sex was defined as those in which a condom was used in all or most (more than half) of their sex encounters.
Living situations were defined by stable housing, unstable housing or others. Living in his own house or living with their parents was considered stable housing. Unstable housing was defined when the person lived in a slum, group home, rented room, shelter, residential treatment facility, or was homeless. The other option grouped different answers to living situations in rented houses or apartments, friends’ houses or other forms of housing.
Participants received pre- and post-test counseling and had their blood and oral fluid collected for HIV rapid testing. Positive test results for oral fluid were sent for confirmatory testing at another OSC that performed the rapid diagnostic blood test. Participants who tested positive for HIV were referred to an HIV outpatient clinic.
The outcome variable was the result of the test for HIV (positive or negative) and the independent variables addressed these aspects: (a) social demographic characteristics: age (≤30 or >30 years old); race/self-declared skin color (white, black or mixed); education (middle school, high school or university); marital status (single/separated or married) and living situation (stable or unstable housing or others); (b) history of sexually transmitted infections (STI) and HIV testing; (c) sexual behavior: number of regular and casual partners in the last six months; condom usage; receptive and insertive anal intercourse; concurrency of sexual partners; anonymous sex in the first encounter, use of alcohol or illegal drugs; paying to have sexual intercourse; HIV-positive sexual partner; (d) self-assessment chances of acquiring HIV (score of 0–10, with 10 as more likely).
Variables were recategorized for analysis according to their distributions and/or occurrence of low cell count. Condom use has often been considered in situations in which it was used in more than half of the sexual intercourses, and rarely in less than half of the sexual intercourses.
After an initial descriptive analysis, we performed a univariate analysis by using Pearson's Chi-square Test to verify the association between variables and HIV infection. Those variables with a p-value less than or equal to 0.20 were included in the multiple logistic regression. Two logistic regression models were developed, one for receptive and another for insertive anal intercourse. Final models were selected by backward methodology at the 0.05 significance level. The Statistical Package Software for Social Science (SPSS) version 17.0 was used for data analysis.
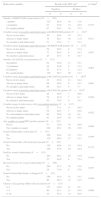
ResultsDuring the 15 months of the study, 341 volunteers were included, representing 49% of the initial sample. However, two volunteers refused to take the HIV test, despite having responded the questionnaire, bringing the total number of people tested to 339. The sociodemographic characteristics of the participants in the survey are shown in Table 1. The majority of respondents were of mixed race (149/43.7%), with higher education and/or incomplete/complete graduate school (197/57.8%), single or separated (274/80.4%), with a mean age of 30.6 years (Table 1).
Sociodemographic information, sexually transmitted infections history, and HIV testing in a group of men who have sex with men in Rio de Janeiro, 2014/2015.
| Variables | N | % |
|---|---|---|
| Age group | ||
| ≤30 years old | 203 | 59.5 |
| >30 years old | 138 | 40.5 |
| Race/color | ||
| White | 124 | 36.4 |
| Black | 68 | 19.9 |
| Mixed | 149 | 43.7 |
| Living situation | ||
| Unstable housing | 47 | 13.8 |
| Stable housing | 191 | 56.0 |
| Other | 103 | 30.2 |
| Relationship | ||
| Single or separated | 274 | 80.4 |
| Married | 67 | 19.6 |
| Schooling | ||
| Middle school (complete or incomplete) | 29 | 8.5 |
| High school (complete or incomplete) | 115 | 33.7 |
| University (complete or incomplete) | 197 | 57.8 |
| Sexually transmitted infections (last 12 months) | ||
| No | 275 | 80.6 |
| Yes | 66 | 19.4 |
| HIV test at least once in life | ||
| No | 82 | 24.3 |
| Yes | 256 | 75.7 |
| Number of HIV tests | ||
| Once | 66 | 19.5 |
| > than 1 time | 190 | 56.2 |
| Never | 82 | 24.3 |
| Someone told you about the possibility of you having HIV | ||
| No | 253 | 74.9 |
| Yes | 85 | 25.1 |
According to socio-economic information, there were a high number of non-respondents for the variable “monthly budget”, but between those who answered (n=190/55.7%), the average was BRL $3111.33 with a median of BRL $2000.00. The majority of the volunteers had stable housing (191/56%), and 13.8% (n=47) resided in unstable housing (Table 1).
A history of STI in the last 12 months was reported by 19.4% (n=66) of the volunteers and 75.7% (n=256) had been tested for HIV at least once in their life time. Out of these, the majority (190/56.2%) had been tested two or more times. A total of 25.1% (n=85) of the participants said that someone had already mentioned that he could be HIV-positive (Table 1).
In general, the participants reported an average of two regular sexual partners in the last six months, and 65% (n=139) reported having a sexual partner. The use of condoms with these regular partners was confirmed by the majority of the respondents, both for receptive (84/40.2%) and for insertive (87/41.6%) anal intercourse (Table 2).
Sexual behavior information in a men who have sex with men group in Rio de Janeiro, 2014/2015.
| Behavioral variables | N | % |
|---|---|---|
| Number of REGULAR sexual partners | ||
| 1 partner | 139 | 43.7 |
| >1 partner | 75 | 23.6 |
| No regular partner | 104 | 32.7 |
| Condom usage in receptive anal intercourse with REGULAR partners | ||
| Never or few times | 66 | 21.1 |
| Always or many times | 84 | 26.8 |
| No receptive anal intercourse | 59 | 18.8 |
| No regular partner | 104 | 33.2 |
| Condom usage in insertive anal intercourse with REGULAR partner | ||
| Never or few times | 68 | 21.7 |
| Always or many times | 87 | 27.8 |
| Non insertive anal intercourse | 54 | 17.3 |
| No regular partner | 104 | 33.2 |
| Number of CASUAL sexual partners | ||
| ≤2 partners | 72 | 22.8 |
| 3–6 partners | 52 | 16.5 |
| ≥7 partners | 40 | 12.7 |
| Non casual partner | 152 | 48.1 |
| Condom usage in receptive anal intercourse with CASUAL partners | ||
| Never or few times | 32 | 10.3 |
| Always or many times | 76 | 24.4 |
| Non receptive anal intercourse | 52 | 16.7 |
| No casual partner | 152 | 48.7 |
| Condom usage in insertive anal intercourse with CASUAL partners | ||
| Never or few times | 35 | 11.2 |
| Always or many times | 86 | 27.6 |
| Non insertive anal intercourse | 39 | 12.5 |
| No casual partner | 152 | 48.7 |
| Condom usage in intercourse with concomitant partners | ||
| Never or few times | 38 | 12.2 |
| Always or many times | 65 | 20.8 |
| Non concomitant sexual intercourse | 106 | 34.0 |
| No regular partner | 103 | 33.0 |
| Any regular or casual HIV-positive partner | ||
| Never | 258 | 77.5 |
| Yes, regular or casual | 75 | 22.5 |
| Sexual relationship on first date | ||
| No | 126 | 37.8 |
| Yes | 207 | 62.2 |
| Sexual relationship with anonymous people | ||
| No | 135 | 43.1 |
| Yes | 178 | 56.9 |
| Paid for sexual relationship | ||
| No | 307 | 92.2 |
| Yes | 26 | 7.8 |
| Received money for sexual relationship | ||
| No | 299 | 90.1 |
| Yes | 33 | 9.9 |
| Sexual relationship drunk or drugged | ||
| No | 222 | 66.7 |
| Yes | 111 | 33.3 |
| Chances of acquiring AIDS (self-assessment) | ||
| 0–2 | 184 | 55.3 |
| 3–5 | 111 | 33.3 |
| 6–10 | 38 | 11.4 |
| HIV test resulta | ||
| Negative | 292 | 86.1 |
| Positive | 47 | 13.9 |
| Testing location | ||
| Civil Society Organization 1 | 117 | 34.3 |
| Civil Society Organizations 2 | 197 | 57.8 |
| Primary Health Unit | 27 | 7.9 |
aTest not performed in two people.
As for the casual relationships, the average was five partners. It was observed that 43.9% (n=72) had one or two sexual partners and 31.7% (n=52) had three to six partners. The condom usage in receptive anal intercourse was confirmed by 47.5% (n=76) and in insertive by 53.8% (n=86). With concomitant sexual partners the condom was used most of the time (65/20.8%) (Table 2).
Most reported having sex in the first date (207/62.2%), and with anonymous partners (178/56.9%), but denied having payed (307/92.2%) or received money for sex (299/90.1%), and not using alcohol or drugs during sex (222/66.7%). The self-assessment chance of getting AIDS, received a low score by the majority, 55.3% (n=184) considering their own chance of acquiring the disease between 0 and 2, and 33.3% (n=111) between 3 and 5 (scores ranging from 0 to 10) (Table 2).
The prevalence of HIV in the MSM study sample was 13.9% (Table 2). The groups with positive and negative results for HIV had similar age and race/color. However, STI history in the last 12 months, someone may have said that he could have HIV, relationship with casual partners with receptive and insertive anal intercourse both without a condom, concomitant relationship with regular and/or casual partners without a condom, HIV-positive partners, and a high score for the self-assessment chance of acquiring HIV were significantly different between HIV-infected and uninfected groups (Tables 3 and 4).
Information on socioeconomic and behaviors in a group of men who have sex with men in Rio de Janeiro according to HIV test result, 2014/2015.
| Variables | Result of the HIV testa | p-Valueb | |||
|---|---|---|---|---|---|
| Negative | Positive | ||||
| N | % | N | % | ||
| Age group | |||||
| ≤30 years old | 174 | 85.7 | 29 | 14.3 | 0.784 |
| >30 years old | 118 | 86.8 | 18 | 13.2 | |
| Race/color | |||||
| White | 107 | 87.7 | 15 | 12.3 | 0.822 |
| Black | 58 | 85.3 | 10 | 14.7 | |
| Mixed | 127 | 85.2 | 22 | 14.8 | |
| Living situation | |||||
| Unstable housing | 37 | 78.7 | 10 | 21.3 | 0.056 |
| Stable housing | 171 | 90.0 | 19 | 10.0 | |
| Other | 84 | 82.4 | 18 | 17.6 | |
| Relationship | |||||
| Single or separated | 239 | 87.5 | 34 | 12.5 | 0.127 |
| Married | 53 | 80.3 | 13 | 19.7 | |
| Schooling | |||||
| Middle school (complete or incomplete) | 23 | 79.3 | 6 | 20.7 | 0.421 |
| High school (complete or incomplete) | 96 | 85.0 | 17 | 15.0 | |
| University (complete or incomplete) | 173 | 87.8 | 24 | 12.2 | |
| Sexually transmitted infections (last 12 months) | |||||
| No | 243 | 88.7 | 31 | 11.3 | 0.005 |
| Yes | 49 | 75.4 | 16 | 24.6 | |
| HIV test at least once in life | |||||
| No | 69 | 85.2 | 12 | 14.8 | 0.735 |
| Yes | 221 | 86.7 | 34 | 13.3 | |
| Number of HIV tests | |||||
| Once | 56 | 84.8 | 10 | 15.2 | 0.834 |
| > than 1 time | 165 | 87.3 | 24 | 12.7 | |
| Never | 69 | 85.2 | 12 | 14.8 | |
| Someone told you about the possibility of you having HIV | |||||
| No | 222 | 88.4 | 29 | 11.6 | 0.050 |
| Yes | 68 | 80.0 | 17 | 20.0 | |
Sexual behavior information in a group of men who have sex with men in Rio de Janeiro according to HIV test result, 2014/2015.
| Behavioral variables | Result of the HIV testa | p-Valueb | |||
|---|---|---|---|---|---|
| Negative | Positive | ||||
| N | % | N | % | ||
| Number of REGULAR sexual partners (N=316) | |||||
| 1 partner | 122 | 88.4 | 16 | 11.6 | 0.572 |
| >1 partners | 63 | 84.0 | 12 | 16.0 | |
| No regular partner | 87 | 84.5 | 16 | 15.5 | |
| Condom usage in receptive anal intercourse with REGULAR partner (N=213)c | |||||
| Never or few times | 56 | 84.8 | 10 | 15.2 | 0.817 |
| Always or many times | 74 | 88.1 | 10 | 11.9 | |
| No receptive anal intercourse | 51 | 87.9 | 7 | 12.1 | |
| Condom usage in insertive anal intercourse with REGULAR partner (N=213)c | |||||
| Never or few times | 59 | 86.8 | 9 | 13.2 | 0.567 |
| Always or many times | 77 | 89.5 | 9 | 10.5 | |
| No insertive anal intercourse | 45 | 83.3 | 9 | 16.7 | |
| Number of CASUAL sexual partners (N=315) | |||||
| ≤2 partners | 62 | 84.9 | 11 | 15.1 | 0.963 |
| 3–6 partners | 44 | 84.6 | 8 | 15.4 | |
| ≥7 partners | 35 | 87.5 | 5 | 12.5 | |
| No casual partner | 130 | 86.7 | 20 | 13.3 | |
| Condom usage in receptive anal intercourse with CASUAL partners (N=165)d | |||||
| Never or few times | 21 | 65.6 | 11 | 34.4 | 0.001 |
| Always or many times | 68 | 89.5 | 8 | 10.5 | |
| No receptive anal intercourse | 48 | 92.3 | 4 | 7.7 | |
| Condom usage in insertive anal intercourse with CASUAL partner (N=165)d | |||||
| Never or few times | 28 | 80.0 | 7 | 20.0 | 0.049 |
| Always or many times | 79 | 91.9 | 7 | 8.1 | |
| No insertive anal intercourse | 30 | 76.9 | 9 | 23.1 | |
| Condom usage in intercourse with concomitant partners (N=312) | |||||
| Never or few times | 27 | 71.1 | 11 | 28.9 | 0.022 |
| Always or many times | 58 | 89.2 | 7 | 10.8 | |
| No concomitant sexual intercourse | 96 | 90.6 | 10 | 9.4 | |
| No regular partner | 87 | 84.5 | 16 | 15.5 | |
| Any regular or casual HIV-positive partner (N=331) | |||||
| Never | 57 | 76.0 | 18 | 24.0 | 0.004 |
| Yes. regular or casual | 228 | 89.1 | 28 | 10.9 | |
| Sexual relationship on first date (N=331) | |||||
| No | 106 | 84.8 | 19 | 15.2 | 0.594 |
| Yes | 179 | 86.9 | 27 | 13.1 | |
| Sexual relationship with anonymous people (N=311) | |||||
| No | 120 | 89.6 | 14 | 10.4 | 0.133 |
| Yes | 148 | 83.6 | 29 | 16.4 | |
| Paid for sexual relationship (N=331) | |||||
| No | 263 | 86.2 | 42 | 13.8 | 0.819 |
| Yes | 22 | 84.6 | 4 | 15.4 | |
| Received money for sexual relationship (N=330) | |||||
| No | 260 | 87.2 | 38 | 12.8 | 0.057 |
| Yes | 24 | 75.0 | 8 | 25.0 | |
| Sexual relationship drunk or drugged (N=331) | |||||
| No | 189 | 85.9 | 31 | 14.1 | 0.886 |
| Yes | 96 | 86.5 | 15 | 13.5 | |
| Chances of acquiring AIDS (self-assessment) (N=331) | |||||
| 0–2 | 171 | 94.0 | 11 | 6.0 | 0.000 |
| 3–5 | 90 | 81.1 | 21 | 18.9 | |
| 6–10 | 24 | 63.2 | 14 | 36.8 | |
Although some risk factors were not statistically significant, it is worth mentioning that there was a greater proportion of positive cases among those living in unstable housing, married or cohabiting, who had sex with anonymous people, and received money for sex.
In all, eleven variables with the p-value less than 0.20 were included in the multivariate logistic regression models. The final two models differ by the variable type of anal intercourse if insertive or receptive. Both models showed that volunteers who had sexual partners (regular or casual) who were HIV-positive and had a high self-assessment chance of becoming infected with HIV were more likely to have the HIV infection, significantly (p-value <0.05). Model 1 showed that those who did not use condoms (never/rarely) in receptive anal intercourse with casual partners had a chance 4.23 times higher of a HIV infection than those who did not report receptive anal intercourse. While, in model 2, it was observed that those who used condoms or not in insertive anal intercourse with casual partners were less likely to acquire HIV compared with those who did not have insertive anal intercourse (Table 5).
Final multivariate models for HIV infection in a men who have sex with men group. considering individual characteristics, in Rio de Janeiro, 2014/2015.
| Variables | OR | IC 95% | OR Adjust. | IC 95% |
|---|---|---|---|---|
| Model 1 | ||||
| Any regular or casual HIV-positive partner | ||||
| Never | 1.00 | – | 1.00 | – |
| Yes. regular or casual | 2.57 | 1.33–4.97 | 2.59 | 1.25–5.36 |
| Condom usage in receptive anal intercourse with CASUAL partner | ||||
| Never or few times | 6.29 | 1.79–22.03 | 4.23 | 1.13–15.81 |
| Always or many times | 1.41 | 0.40–4.96 | 1.22 | 0.33–4.49 |
| No receptive anal intercourse | 1.00 | – | 1.00 | – |
| No casual partner | 1.85 | 060–5.68 | 2.12 | 0.66–6.79 |
| Chances of acquiring AIDS (self-assessment) | ||||
| 0–2 | 1.00 | – | 1.00 | – |
| 3–5 | 3.63 | 1.68–7.86 | 3.22 | 1.40–7.43 |
| 6–10 | 9.07 | 3.70–22.26 | 7.37 | 2.76–19.70 |
| Model 2 | ||||
| Any regular or casual HIV-positive partner | ||||
| Never | 1.00 | – | 1.00 | – |
| Yes. regular or casual | 2.57 | 1.33–4.97 | 2.57 | 1.24–5.32 |
| Condom usage in insertive anal intercourse with CASUAL partner | ||||
| Never or few times | 0.83 | 0.27–2.54 | 0.38 | 0.11–1.30 |
| Always or many times | 0.30 | 0.10–0.86 | 0.22 | 0.07–0.70 |
| No insertive anal intercourse | 1.00 | – | 1.00 | – |
| No casual partner | 0.51 | 0.21–1.24 | 0.51 | 0.20–1.31 |
| Chances of acquiring AIDS (self-assessment) | ||||
| 0–2 | 1.00 | – | 1.00 | – |
| 3–5 | 3.63 | 1.68–7.86 | 3.56 | 1.54–8.21 |
| 6–10 | 9.07 | 3.70–22.26 | 10.55 | 3.85–28.88 |
OR, odds ratio; IC, confidence interval; OR Adjust., adjusted odds ratio.
The HIV prevalence in MSM in this study was 13.9%. These results are consistent with other studies that identified a higher prevalence of HIV in the MSM population. Data from a meta-analysis of six Brazilian studies indicated a combined HIV prevalence of 13.6% among MSM.14 Another research conducted in 10 Brazilian cities showed an HIV prevalence of 14.2% among MSM.23
The findings of the study showed that the type of sexual practice is associated with HIV infection. In both models we found that having sexual intercourse with regular or casual partners known to be HIV positive has greater risk for the disease, accounting for about 2.5-fold the chance of those who have never had a positive partner. Model 1 showed that the chance of acquiring HIV in unprotected receptive anal intercourse with casual partners was 4.2-fold higher than those who did not practice receptive anal intercourse. Among those who had a protected act, the chance of HIV infection was 1.22-fold higher than those who did not have this kind of anal intercourse. Studies have assessed unprotected anal sex among MSM infected with HIV; there is great variability in the nature of the relationship, stable or casual, and also the sexual practice, receptive or insertive.14,24,25 Rocha et al.26 found that the practice of receptive anal sex without a condom among MSM was related to those who lived with a male partner and had a stable relationship. The findings of a Brazilian study in MSM showed that the main reasons for not having taken an HIV test were: believing in the trustworthiness of his partner, fear of suffering discrimination in case of a positive test result, belief that there was no risk of becoming infected as he had a regular partner.27
A study conducted in Central Asia found that 69% of MSM reported unprotected anal sex, and this practice was associated with an increased risk of HIV infection.28
In model 2 insertive anal intercourse with casual partners with or without condom showed a lower chance of HIV infection compared to those with no practice of insertive anal intercourse. It is important to note that the chance of HIV infection among those who had protected anal intercourse (with a condom) was 78% lower than those who did not have insertive anal intercourse. However, unprotected acts (without condom) the chance of HIV infection was 62% lower than for those who did not have this kind of sexual practice. In other words, the findings of this study indicate insertive intercourse can provide some kind of protection from HIV infection.
This result may be related to sexual behavior strategies for risk management of HIV infection adopted by MSM. Unprotected anal sexual practices in the insertive position among MSM presented lower risk of contracting an infection of HIV in comparison with those that adopted the receptive position.29,30
The choice of sexual practice and partner depends both on the risk of acquiring or transmitting an STD and the benefits of sexual intercourse. In this sense, research has shown that MSM have used different risk reduction strategies to protect themselves and their partners of HIV.31,32 The term “seroadaptation” has been used to define, broadly, one set of risk reduction practices based on the knowledge of HIV status for him and his partner to decide during intercourse.33–35
Sexual position as a risk management strategy for HIV infection among MSM has been previously reported.19,36 Terto Jr37 emphasized the incorporation of sexual practices to reduce the chances of becoming infected or transmitting HIV among MSM. One of the strategies cited by the author was the seropositioning regarding the choice of sexual practices such as receptive anal sex without a condom only with HIV-negative sexual partners and anal insertive sex with strangers or HIV-positive partners. The risk of a MSM who are in the insertive position at intercourse to become HIV infected is smaller than for those who adopt a receptive role.29,30,38 Another study found that infected MSM use HIV transmission reduction strategies, adopting seroposition practices when the HIV-positive partner does not take a position during insertive unprotected anal sex.39
Serosorting has also been a practice adopted by MSM, in which the choice of sexual partners or using condoms is done selectively based on serological HIV status. A study conducted in the United States noted that most HIV-infected MSM who had unprotected anal sex seek to limit the risk of HIV transmission to their partners using strategies like serosorting and strategic positioning, preferring to take the receptive role.40
Jin et al.41 in a survey of MSM who had unprotected anal sex practices identified a lower likelihood of HIV infection among those who practiced some risk reduction behavior, such as serosorting or seropositioning when compared to those who did not adopt this strategies. However, it is important to stress that MSM who had unprotected anal sex with risk reduction had a risk to be HIV infected about three times higher compared to those who did not practice unprotected anal intercourse.
Models 1 and 2 showed that individuals who self classified themselves with high chances of becoming HIV infected (score 6–10) are actually those who had a greater chance of infection, about seven (Model 1) to 10 times (Model 2) the chance of infection for those who considered themselves to have very low risk (score 0–2). However, it should be emphasized that even those who considered at low risk of HIV acquisition (score 3–5) also had a high chance of HIV infection, about three times the chance compared to individuals self classified as very low (score 0–2).
Despite the increased risk of HIV infection in the MSM population, a study in 2007 in Mato Grasso do Sul State indicated that MSM do not perceive themselves as vulnerable to HIV infections. Among respondents, only 27% have partaken in risky behavior, which is inconsistent with reports about condom use and number of partners. It is observed that 65% of gay men, 75% of bisexuals, and 33% of transvestites reported at least five partners in the last month. Of this group, 59% had more than 11 partners, and 35% said they did not use condoms in all sexual intercourses.42
Some behaviors and attitudes classically considered to be associated with higher risk for HIV infection did not show statistical significance (p<0.05) in the final multiple logistic regression models. It is worth noting that this result may have been influenced by the sample size of the study that may have not been sufficient to show associations with statistical significance. However, there is relevance to the study because it represents a sample of high HIV prevalence, despite the small sample size. This does not limit the generalization of the results. In addition, access to MSMs for epidemiological studies is difficult, which is translated by the scarcity of studies in MSM in Brazil, underscoring the importance of the current study.
It is noteworthy that the univariate analysis showed a statistical association between HIV infection with the variables living situation and marital status; STI history in the last 12 months; someone assuming he could have HIV; concomitant relationship with regular or casual partners without a condom; and receiving money for sex.
These findings corroborate the results of recent research. The living situation (stable or unstable housing or others), which was used as a proxy for socioeconomic status was associated with HIV infection. Studies have shown increased morbidity and mortality from AIDS in socioeconomically disadvantaged populations.3,43
The risk for HIV infection among those with concomitant sexual partners was also reported by Tieu et al.44 in a study conducted in New York City (USA). Men had an average of 3.2 male partners, 63.2% reported concurrent sexual partners, and 71.5% believed that their partners had concurrent partners.
Studies have shown an association with sexual activity without condom use among MSM in stable relationships and living in marital status.24,26,45 These results corroborate the findings of the present study that showed an increased risk of HIV acquisition among married MSM.
One limitation of this study is related to the inclusion criteria, as participants should have knowledge of their HIV status or state they negative for HIV. However, this was a cross-sectional study in which all measurements were performed in a single point in time and one could not be sure that all volunteers were indeed negative at study entry. The questionnaire responses from people known to be positive for HIV could bias our final results. But the majority of the participants took the HIV test in the two OSC without any healthcare. Therefore, we believe that participants were probably under high risk but were unaware of their serostatus.
It is also worth noting that the form of selecting participants, through invitations to those MSM who had been to OSC or in other venues for other activities, may have had some selection bias. Our study faced difficulty in selecting MSM volunteers. The great majority was selected directly at testing study sites which probably resulted in the selection of high risk subjects.
Traditionally, epidemiology has been concerned with the study of risk behavior at the individual level. But in that way, it does not consider the context of interactions in networks of groups of people with some common characteristics. The spread of sexually transmitted infections depends on interaction for acquiring an infection and is related to the choice of partner and sexual practices.46–48 In this sense, the next step of the study is to analyze the network of sexual partners, since some variables related to partnerships were significantly associated to the HIV status in univariate analysis.
ConclusionIn conclusion, the study shows the MSM population had a higher risk of HIV infection compared to other population subgroups. This fact is related to practices and high risk sexual behaviors as well as specific sociodemographic characteristics that make MSM a vulnerable group for the disease. The study showed low consistent condom use in regular and casual sex. Moreover, it indicates the use of infection risk reduction strategies HIV such as seropositioning. The use of risk reduction strategies among MSM based on knowledge of HIV status of their own and of their partner may be ineffective.
In this context, it remains a challenge to reduce the transmission of HIV among MSM, requiring the proposal of policies and prevention strategies for HIV/AIDS directed specifically to this population subgroup.
FundingThis research was funded by the Ministry of Health's Department of Public Health Control, Prevention and Monitoring of sexually transmitted infections, AIDs and Viral Hepatitis (DDAHV/SVS/MS) in collaboration with the United Nations’ Office on Drugs and Crime (UNODC).
AuthorshipRMC Torres participated in the study design, analysis, data interpretation and drafting of the article. MM Cruz contributed to the critical review and approval of the final version of the manuscript. ARS Périssé contributed to the study design, analysis, and interpretation of data, as well as approval of the final revision of the manuscript. All authors assume responsibility for all aspects of the work. DRF Pires participated in study implementation and the critical review and approval of the final version of the manuscript.
Conflicts of interestThe authors declare no conflicts of interest.
To the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (Capes) for the financial support through an education grant for the graduate school. To the Public Health Doctorate Program of ENSP/Fiocruz. To the Secretaria de Estado de Saúde do Rio de Janeiro (SES-RJ). To the DDAHV/SVS/MS and the UNODC for the financial support of the project. To the Laboratório de Pesquisa Clínica em DST e AIDS of Instituto Nacional de Infectologia Evandro Chagas (INI/Fiocruz) for the essential partnership for the development and implementation of the study.