Coagulase-negative Staphylococcus has been identified as the main nosocomial agent of neonatal late-onset sepsis. However, based on the pharmacokinetics and erratic distribution of vancomycin, recommended empirical dose is not ideal, due to the inappropriate serum levels that have been measured in neonates. The aim of this study was to evaluate serum levels of vancomycin used in newborns and compare the prediction of adequate serum levels based on doses calculated according to mg/kg/day and m2/day. This is an observational reprospective cohort at a referral neonatal unit, from 2011 to 2013. Newborns treated with vancomycin for the first episode of late-onset sepsis were included. Total dose in mg/kg/day, dose/m2/day, age, weight, body surface and gestational age were identified as independent variables. For predictive analysis of adequate serum levels, multiple linear regressions were performed. The Receiver Operating Characteristic curve for proper serum vancomycin levels was also obtained. A total of 98 patients received 169 serum dosages of the drug, 41 (24.3%) of the doses had serum levels that were defined as appropriate. Doses prescribed in mg/kg/day and dose/m2/day predicted serum levels in only 9% and 4% of cases, respectively. Statistical significance was observed with higher doses when the serum levels were considered as appropriate (p<0.001). A dose of 27mg/kg/day had a sensitivity of 82.9% to achieve correct serum levels of vancomycin. Although vancomycin has erratic serum levels and empirical doses cannot properly predict the target levels, highest doses in mg/kg/day were associated with adequate serum levels.
The introduction of new technologies in neonatal units has caused high nosocomial infection rates with subsequent complications among newborns. Coagulase-negative Staphylococcus has been identified as the main nosocomial agent, affecting more than 50% of all cases of late-onset neonatal sepsis.1–3
Vancomycin is widely used for confirmed or suspected neonatal sepsis as around 90% of coagulase negative Staphylococci strains are oxacillin and penicillin-resistant.4,5 However, toxicity, pharmacokinetic variability and empirical doses used for newborns are important topics related to vancomycin use in this population.6–11 Measurement of area under the curve to achieve minimum inhibitory concentration (MIC) against Staphylococci is an important parameter to therapeutics with this drug.6,7
The usual empirical dose for the neonatal population ranges from 10 to 15mg/kg/dose, administered one to four doses a day.1,3,5,8 However, studies in pediatric population have reported on the need for higher doses of vancomycin, up to 60mg/kg/day, in order to achieve the desired serum level.6,7 A minimum recommended through serum level of at least 5–10mcg/mL is required for effective therapy and fewer side effects, including nephrotoxicity.8–10 Higher concentration targets ranging from 15 to 20mcg/mL are recommended even for children with more severe infections such as osteomyelitis and meningitis.6,11
However, empirical dose of vancomycin is not ideal according to inappropriate serum levels of the drug that have been reported in neonates in several studies.5,9,10,12,13 Although there have been previous descriptions of dose calculations based on nomograms and adjustments according to drug clearance and the patient's renal function,5,14–17 no study has analyzed doses that were prescribed according to body surface (m2/day) and the resulting serum vancomycin levels. We hypothesized that higher serum levels could be achieved based on doses calculated according to body surface.
The present study was conducted in order to evaluate serum levels of vancomycin that were used to treat late-onset sepsis in newborns and compare the prediction of adequate serum levels based on doses calculated according to mg/kg/day and m2/day.
Material and methodsDesignThis is an observational retrospective cohort, real life study, held at the Neonatal Progressive Care Unit (NPCU) of the Hospital das Clinicas, Federal University of Minas Gerais, from January 2011 to June 2013.
Inclusion criteriaNewborns who were admitted to the NPCU, diagnosed with Healthcare Associated Infections (HAI), and treated with vancomycin for the first episode of late-onset sepsis (defined as sepsis after 48h of age), were included in this study if they had vancomycin level tested as recommended.
Data collectionInformation was collected through prospective and active surveillance of infants at risk by trained professionals of the Hospital Infection Control Commission (HICC). Standardized information about gestational age at delivery, birth weight, gender, data and criteria for diagnosis and treatment of bloodstream infection are routinely registered.
Clinical or laboratory-confirmed bloodstream infection followed the criteria of the Agência Nacional de Vigilância (ANVISA) based on National Healthcare Safety Network of the Centers for Diseases Control and Prevention recommendations.
- a)
Clinical Bloodstream Infection – at least one of clinical signs (thermal instability, apnea, bradycardia, food intolerance, worsening respiratory distress, glucose intolerance, hemodynamic instability, and hypoactivity or lethargy) AND all of following criteria: hemogram with three altered parameters or increased C-Reactive protein; not performed or negative blood culture; no evidence of infection at another site; antimicrobial therapy initiated or sustained by the attending physician.
- b)
Laboratory-confirmed bloodstream infection – at least one of clinical signs (thermal instability, apnea, bradycardia, food intolerance, worsening respiratory distress, glucose intolerance, hemodynamic instability and hypoactivity or lethargy) not related to an infection at another site AND at least one of the following:
- -
common skin contaminant cultured from two or more blood cultures drawn on separate occasions;
- -
common skin contaminant (e.g., diphtheroids, Bacillus sp., Propionibacterium sp., coagulase-negative staphylococci, or micrococci) cultured from at least one blood culture from a patient with an intravascular line, and the physician institutes appropriate antimicrobial therapy.
- -
Information were collected daily and systematically included in the HICC internal program for further analysis.
Main outcomeVancomycin levels were always collected before next dose, corresponding to through levels. Adequate serum levels of vancomycin were considered as those between 10 and 20mg/dL. According to recent studies and considering that neonatal sepsis is a severe event, levels below 10mg/dL were considered as decreased and inappropriate while those above 20mg/dL were defined as increased and inappropriate.6,9,10
Statistical analysisStatistical analysis was performed with the Statistical Package for Social Sciences (SPSS Inc., USA), version 19.0.
Total dose in mg/kg/day, dose/m2/day, age, weight, body surface and gestational age at delivery were independent variables in the predictive model of adequate serum levels in simple linear regression. Statistical significance was considered when p<0.05. Multiple linear regression was performed with all variables with p<0.05 in univariate linear regression.
Total daily doses of vancomycin was considered as proposed by previous published studies6,7,10,11,13,17 because neonatal doses can vary according to birth weight and post-gestational age.
Doses were compared among groups according to appropriate (group 1) and inappropriate (group 2) serum levels, using the Mann–Whitney (when variables presented non-normal distribution) or t-test (when variables presented normal distribution).
The Receiver Operating Characteristic curve (ROC curve) for proper serum vancomycin levels was also obtained.
Ethics considerationsThe study was approved by the Institutional Ethics Committee.
ResultsA total of 98 patients were included in analysis and the mean gestational age was 33.52 weeks (SD=4.41) and 63 patients (64.3%) were premature. Forty-eight (49%) were male and the average age at the start of vancomycin treatment was 18.4 days (SD=16.5).
First measurement of vancomycin levels of 95 patients showed that 28 (28.6%) of them adequate values, 57 (58.2%) had diminished levels (below 10mg/dL) and 10 (10.2%) presented higher levels (above 20mg/dL). Three patients had their first vancomycin dose modified without serum levels of the drug.
During treatment, there were a total of 169 serum measurements of the drug, with an average of 1.72 doses per patient. There were 64 dose modifications based on the serum levels. Thus, the average number of modifications per patient was 0.72 (SD=0.89) with a median of 1, and varying from 1 to 5 times.
Among the 169 measurements, 41 (24.3%), 112 (66.3%), and 16 (9.5%) had serum levels that were defined as adequate, diminished, and above the target value, respectively.
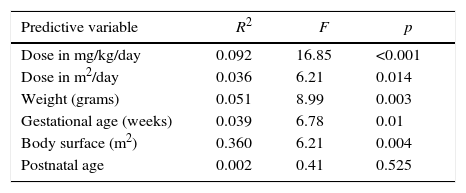
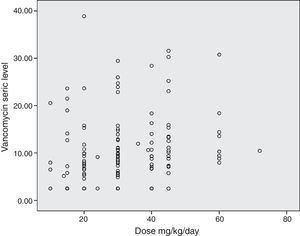
The simple linear regression model showed that doses prescribed in mg/kg/day predicted serum levels in only 9% of cases, but higher doses presented a significant association with high serum vancomycin levels (p<0.001). Doses that were prescribed in dose/m2/day showed similar results, with adequate serum prediction in only 4% of cases. However, a higher statistical association was observed between higher doses and higher serum vancomycin levels (p=0.014). Weight, gestational age, and body surface did not predict adequately the serum levels in multiple regression analysis (Table 1).
Simple linear regression for prediction of adequate serum vancomycin levels, referral Neonatal Unit for Progressive Care, Hospital das Clínicas/UFMG, Belo Horizonte, Brazil, from 2011 to 2013.
| Predictive variable | R2 | F | p |
|---|---|---|---|
| Dose in mg/kg/day | 0.092 | 16.85 | <0.001 |
| Dose in m2/day | 0.036 | 6.21 | 0.014 |
| Weight (grams) | 0.051 | 8.99 | 0.003 |
| Gestational age (weeks) | 0.039 | 6.78 | 0.01 |
| Body surface (m2) | 0.360 | 6.21 | 0.004 |
| Postnatal age | 0.002 | 0.41 | 0.525 |
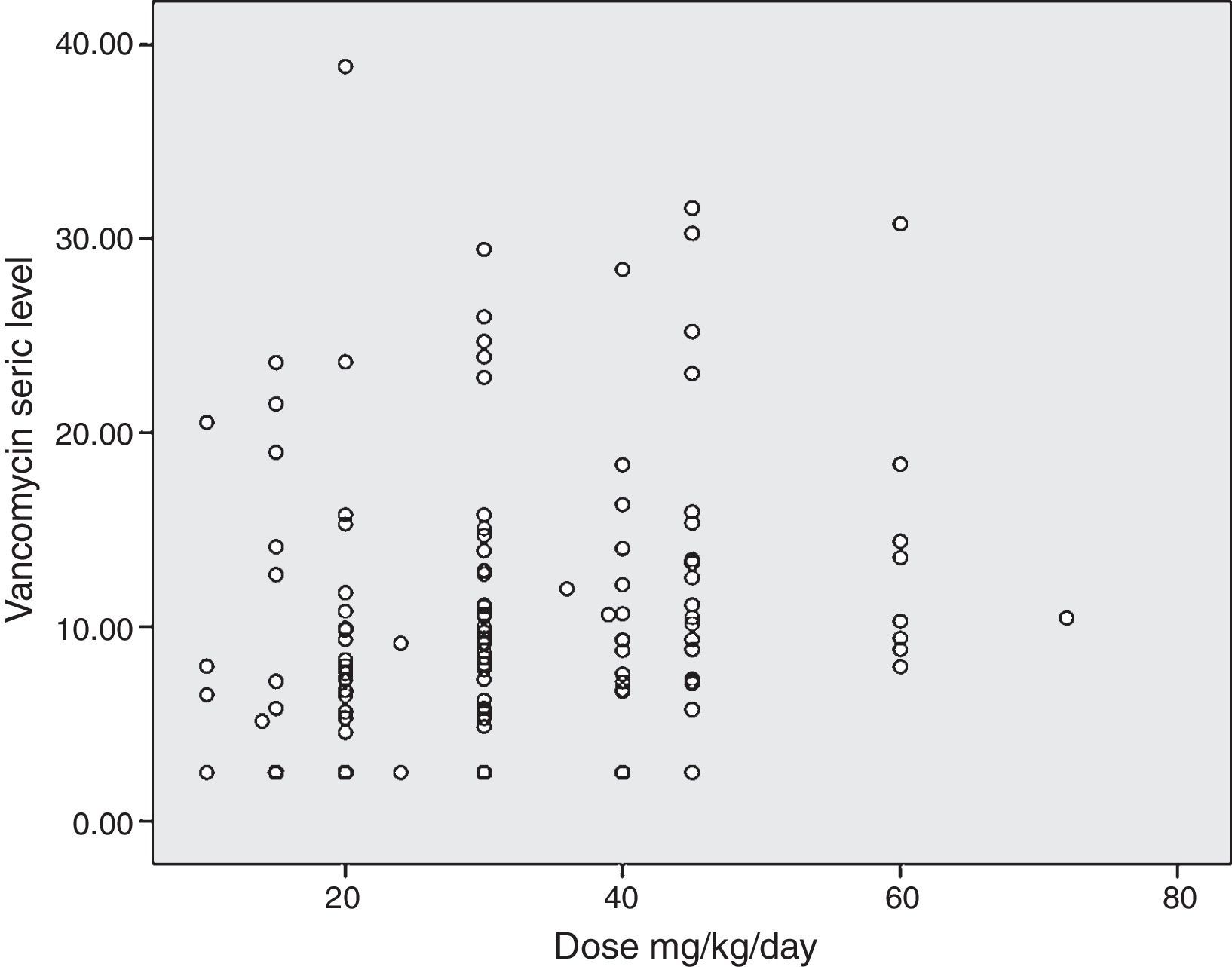
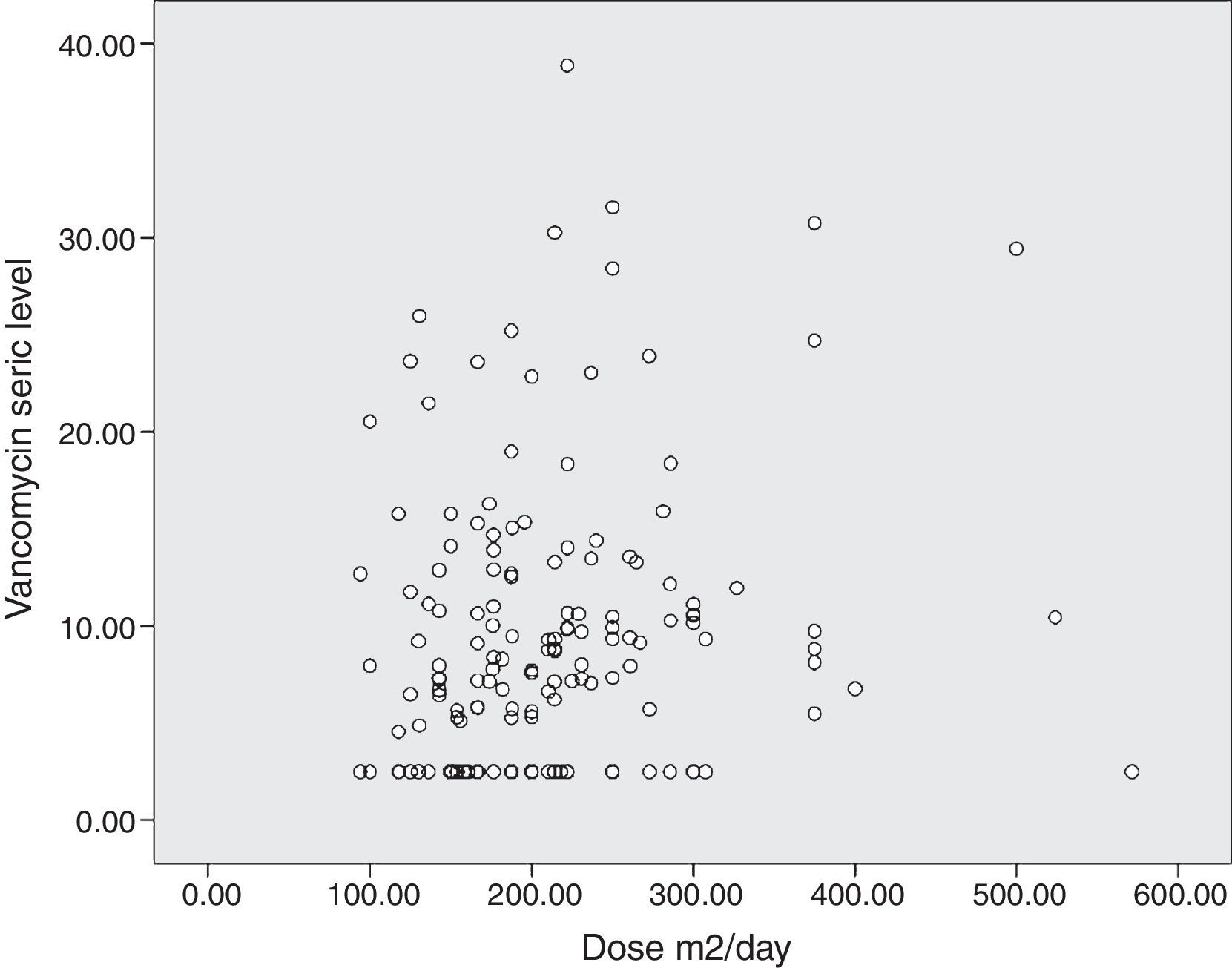
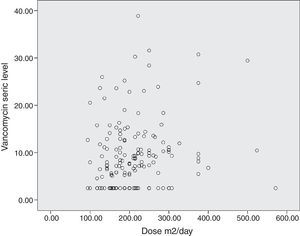
The diagram of dose dispersion in mg/kg/day (Fig. 1) and dose in m2/day (Fig. 2) revealed that although there was no proper prediction with any of the doses for the calculation basis, doses that were calculated in m2/day showed higher homogeneity in graphical distribution.
During multiple linear regression analysis for the prediction of the serum levels, only the prescribed dose in mg/kg/day presented with statistically significant associations (p<0.001).
Statistical differences were observed when dose in mg/kg day was compared among groups (Mann–Whitney with p<0.001) (Group 1 median=36; Group 2 median=27mg/kg/day). However, differences between groups were not observed when compared to dose/m2/day (p=0.213) (Group 1 median=195.6; Group 2 median=194mg/m2/day).
After excluding 16 patients with increased serum levels (outliers), we compared patients with low and adequate serum levels. Mann–Whitney also showed statistically significant differences when compared to total dose in mg/kg/day among groups with adequate and decreased (p<0.001) (Group 1 median=36; Group 2 median=20mg/kg/day).
Statistically significant differences between the serum level values in term (mean 10.91mg/dL, SD 6.88) and preterm infants (average 8.05mg/dL; DP7, 46) were observed. Higher serum levels were achieved in the first group (t-test=2.52; p=0.013).
When comparing the value of the serum level achieved according to days of life, there were no observed differences in serum levels between newborns at ≤7 days (median 9.14, SD=7.22) or >7 days of life (mean 9.16, SD=7.33; t-test=0.12 and p=0.99.1).
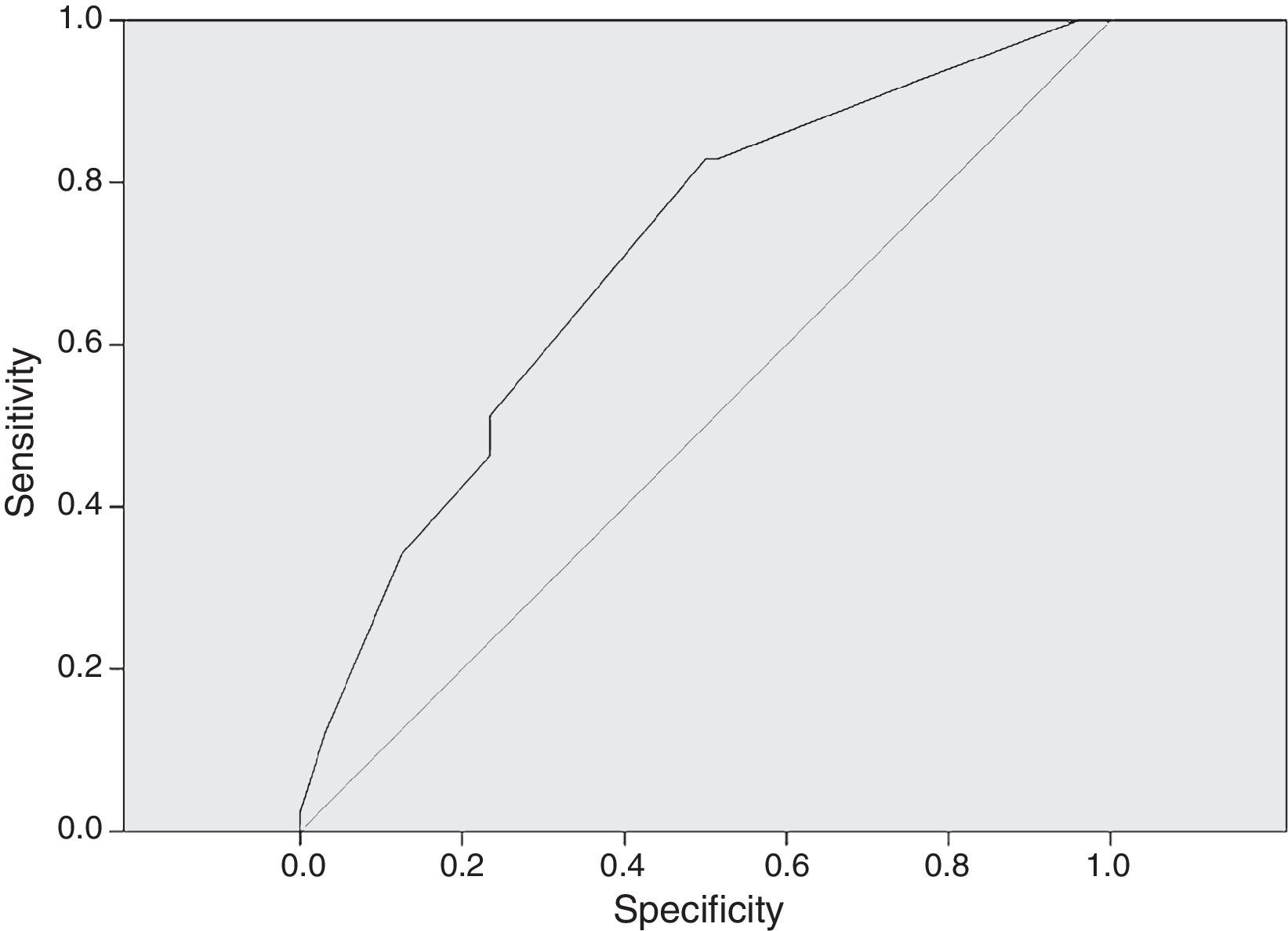
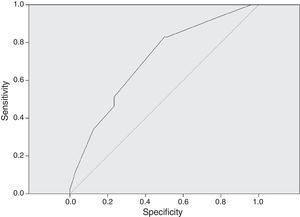
In Fig. 3, the ROC curve for dose in mg/kg/day correlated to adequate serum levels. An AUC of 0.706 was considered as the minimum for a satisfactory (95% CI 0.617–0.795) result. A dose of 27mg/kg/day had a sensitivity of 82.9% to achieve correct serum levels of vancomycin.
DiscussionVancomycin doses in mg/kg/day were not highly predictive of adequate serum levels but higher doses were significantly associated with adequate vancomycin serum levels. Other retrospective studies in the neonatal and pediatric population also showed poor performance of empirical treatments with vancomycin, with adequate target levels of 25–34%, considering trough levels from 10 to 20mg/L.9,10,18 Even in studies that considered baseline serum levels from 5 to 15mg/L, suitable dosages ranged from 31 to 66%.4,12,13,18
In this study, a dose of 27mg/kg/day could be the minimum recommended dose for newborns. Although empirical recommended doses vary from 10 to 15mg/kg, intervals between doses often vary from q48 to q6h, achieving a low percentage of vancomycin target levels.4,10,12,18,19
The main reasons for monitoring the dose of vancomycin by serum concentrations are for maintaining therapeutic levels and preventing ototoxicity and nephrotoxicity.8,20
Considering that vancomycin has an erratic distribution and renal elimination, the duration of action is crucial for achieving and maintaining serum levels which indicated the need for longer dosing intervals. Hoog et al.13 compared the use of 30mg/kg/day at 12-h and 8-h intervals, and a percentage of the appropriate concentration of 95% was achieved when shorter intervals were used. In the model described by Dersch-Mills et al.10 dose adjustments of 10mg/kg every 6hours were necessary in order to reach adequate serum levels in 72% of cases.
Even clinical trials of continuous dose infusion indicated the need of 30mg/kg/day to achieve 75–90% of suitable serum levels.17,21 Zhao et al.15 preformed a prospective study with continuous infusion of vancomycin with doses simulation ranging from 15 to 35mg/kg/day and the authors reported 70.7% of serum levels between 15 and 25mg/L.
In the present study, even higher doses were observed in the group that reached the appropriate level with a median of 36mg/kg/day and a median of one dose modification.
In a retrospective study among pediatric patients with normal renal functions, Glover et al.22 observed the need to modify doses at least once on average (ranging from 1 to 4 times) in order to achieve adequate serum levels, which required up to 60mg/kg/day of vancomycin.
Doses that were administered in m2/day were not investigated in a previous study. Although predicting the serum level was even lower than that achieved by mg/kg/day, the scatter plot diagram revealed a greater homogeneity of measurements. Possibly larger doses are needed in mg/m2 in order to achieve adequate serum levels. The high percentages of body water and extracellular space in newborns lead to lower plasma drugs concentrations that diffuse into these compartments.23,24 Thus, in children aged less than two years, hydrophilic drugs with low volume of distribution should be normalized by body surface,25 and similarly considered for vancomycin.
It is noteworthy that full-term babies had higher serum median levels (10.91mg/dL) compared to preterm newborns but the difference was not significant when adjusting for days of postnatal life. It is known that vancomycin pharmacokinetics is affected by the immaturity of the renal function of preterm infants and drug half-life and clearance are even higher in preterm newborns due to the high percentage of body water, especially during the first postnatal week.26,27
Some authors suggest dose adjustment through nomograms and formulae.28 Studies investigating these methods in the neonatal population should consider the maturation of renal function and vancomycin clearance for dose calculation and adjustment for drug maintenance.4,14–17,29
Doses of up to 60mg/kg/day of vancomycin are recommended for children under one year of age based on a study that reached AUC/MIC >400, which is considered appropriate for the treatment of Staphylococcus aureus.7 Stockmann et al.30 have also considered target concentrations of AUC/MIC >400 in neonates and observed the linear correlation between greater AUC and higher serum levels.
Despite being a study with prospective and active surveillance, there were difficulties related to information collection due to non-standardized procedures related to antibiotics prescription by the clinical staff. Vancomycin serum levels were not always performed as demanded and dose modifications were observed even without monitoring, limiting the number of drug evaluations.
In conclusion, vancomycin has erratic serum levels and empirical doses calculated in mg/kg/day or m2/day cannot properly predict the target levels. Doses based in m2/day showed higher homogeneity. Besides, higher doses based in mg/kg/day were associated with adequate serum levels, even after adjusting for gestational age, weight, and body surface. Considering the influence of these variables on drug distribution, this indicates necessity for higher doses, as at least 30mg/kg/day, in order to achieve the target and therapeutic levels.
FundingInstitutional Program for Scientific Scholarships – Federal University of Minas Gerais (UFMG)/Foundation for Research Support of Minas Gerais State (FAPEMIG) and Research Pro-rectory of Research – Federal University of Minas Gerais.
Conflicts of interestThe authors declare no conflicts of interest
The authors thank Paulo Henrique Orlandi Mourão, for his technical assistance in the HICC internal database.