Toxoplasmic retinochoroiditis (TR) is the most common identifiable cause of posterior uveitis in Brazil. Response to treatment and clinical presentation may vary significantly. We assessed serum levels of brain-derived neurotrophic factor (BDNF), glial cell line-derived neurotrophic factor (GDNF), nerve growth factor (NGF), neurotrophin (NT)-3, and NT-4/5 in patients with active TR, before and after TR treatment.
MethodsTwenty patients with active lesion and 15 healthy controls were enrolled in the study. Serum concentration of neurotrophic factors was determined by enzyme-linked immunosorbent assay.
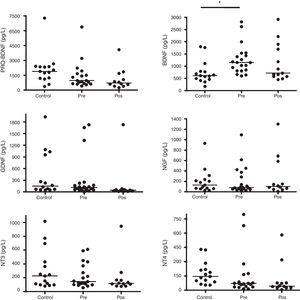
ResultsBDNF levels were significantly higher in patients before treatment when compared with controls (p=0.0015). There was no significant difference in pro-BDNF, NGF, GDNF, NT-3, and NT-4/5 levels between TR patients and controls. Treatment did not affect the levels of these factors.
ConclusionBDNF may be released in the context of the active TR inflammatory response.
Toxoplasmic retinochoroiditis (TR) is the most common identifiable cause of posterior uveitis in Brazil, representing up 85% of all cases.1 TR is caused by the acquired infection with the protozoan Toxoplasma gondii.2 Approximately one third of human population is infected by T. gondii worldwide, but most of seropositive patients do not develop a symptomatic disease.3 In the United States, for instance, the proportion of infected patients who had ocular toxoplasmosis is approximately 2%, but the prevalence of ocular involvement is greater in other parts of the world such as Brazil, where it is around 18%.4–6
Response to treatment and clinical presentation may vary significantly, with some patients presenting one episode of mild inflammation, whereas others have multiple recurrences of severe uveitis that include persistent vitreous opacities, epiretinal membrane, and cystoid macular edema leading to loss of eyesight. The reasons for such clinical heterogeneity remain uncertain.4,7,8
Neurotrophic factors are proteins that aid in the differentiation, survival and maintenance of neurons.9 Retinal glial cells can produce several neurotrophic factors, such as brain-derived neurotrophic factor (BDNF), glial cell line-derived neurotrophic factor (GDNF), and nerve growth factor (NGF) that have neuroprotective effects under pathological conditions.4,7,10
We hypothesized that levels of neurotrophic factors increase during TR activity in parallel with the inflammation of uvea, normalizing after treatment. Therefore, the objective of this study was to investigate the serum levels of BDNF, pro-BDNF, NGF, GDNF, neurotrophin (NT)-3, and NT-4/5 in TR patients, before and after treatment, comparing with control subjects.
Twenty patients with TR (27.5±8.4 years, male/female=8/12) were consecutively recruited from the Department of Ophthalmology, Universidade Federal de Minas Gerais, Belo Horizonte/MG, Brazil. The diagnosis of active TR was based on the presence of a typical clinical presentation, i.e. an active white focal retinal lesion with an associated hyperpigmented chorioretinal scar, and positive serology for T. gondii. Each patient received a detailed ophthalmologic examination. All patients were treated with oral prednisone (1mg/kg/day for one week, followed by tapering doses), pyrimethamine (25mg/day), sulphadiazine (1g four times per day), and folinic acid (7.5mg/day) for 35 days, according to the protocol of the Uveitis Section, Department of Ophthalmology, Universidade Federal de Minas Gerais, Brazil.
Fifteen healthy individuals with positive IgG antibody for T. gondii without any history of ocular disease (31.11±6.64 years, male/female=4/11) were recruited as controls. All controls underwent an ocular examination to exclude the presence of any retinal lesion. No subject was receiving treatment with any immunosuppressive or immunomodulatory drug.
Peripheral blood was collected and centrifuged at 1000×g for 10min. Next, serum was removed and stored at −80°C until analysis. All blood samples from patients were collected twice, i.e. at the moment of clinical diagnosis and after treatment completion.
The concentration of neurotrophic factors in serum of patients and controls was determined by sandwich ELISA kits according to the procedures supplied by the manufacturer (DuoSet, R&D Systems, Minneapolis, MN, USA). All samples were assayed in duplicate. The detection limit for these assays was 10pg/ml.
Analyses were performed using the Statistical Package for the Social Science (SPSS) software version 20.0. For all comparisons, a two-tailed p-value <0.05 was considered significant. As neurotrophic factor measures did not present normal distribution, non-parametric tests were used. Kruskal–Wallis with post hoc tests were carried out to assess median differences among controls and TR groups. Wilcoxon test was performed to compare the results before and after treatment. The study protocol adhered to the tenets of the Declaration of Helsinki and was approved by the local Institutional Review Board.
BDNF levels were significantly higher in TR patients before treatment when compared with controls (p=0.0015). BDNF levels decreased after treatment, but this did not reach statistical significance (Fig. 1). There were no significant differences in Pro-BDNF, NGF, GDNF, NT-3, and NT-4/5 levels between TR patients and controls. Treatment did not affect the levels of these factors.
Brain-derived neurotrophic factor (BDNF), glial cell line-derived neurotrophic factor (GDNF), nerve growth factor (NGF), neurotrophins (NT)-3, and NT-4/5 levels in the serum of patients with active TR, before (PRE) and after treatment (POS). *For all comparisons, p values <0.05 indicate statistical significance.
There was no significant correlation between any neurotrophic factor and levels of visual acuity, grade of vitreous haze, and lesion size. There was no association between clinical improvement and neurotrophic factor changes after treatment.
This is the first study to address circulating neurotrophic factors in TR. Patients with active TR had increased serum levels of BDNF in comparison with controls.
BDNF is the most widely distributed neurotrophin in the CNS and plays a central role in the regulation of neuronal survival mainly by activating through the neurotrophic tyrosine kinase receptor B (TrkB).9 BDNF may be produced following inflammatory stimuli as a compensatory mechanism to minimize neuronal damage. Its sources remain undetermined in the current context, but BDNF may be released by retina glial and neuronal cells and/or infiltrating lymphocytes.7,10,11 Interestingly, higher serum levels of BDNF were also observed in patients with primary open-angle glaucoma, being regarded as a useful biomarker for early detection of this disease.12
Experimental models have indeed described neuroprotective effects of either endogenous or exogenous neurotrophins. For instance, BDNF and NGF inhibited the osmotic swelling of glial (Müller cells) and bipolar cells induced by barium solution in the rat retina cells.10,13 Conversely, BDNF did not prevent photoreceptor degeneration in TrkB knockout mice, while failed to stimulate Müller cells proliferation and expression of neural markers in the degenerating retina.14 One potential source of BDNF is the infiltrating mononuclear cells, especially T lymphocytes. T lymphocytes are needed for complete control of ocular toxoplasmosis.15,16 These cells could produce both cytokines and lytic granules in attempt to eliminate the invading parasite, and BDNF to prevent retina damage.16–18 This tempting hypothesis needs to be confirmed by further studies.
In conclusion, among a series of neurotrophic factors assessed in patients with TR, only BDNF levels were different in comparison with controls. BDNF may be released in the context of the active TR inflammatory response.
FundingThis study was supported by grants from CNPq, and Fapemig, Brazil.
Conflicts of interestThe authors declare no conflicts of interest.