Chagas disease reactivation has been a defining condition for acquired immune deficiency syndrome in Brazil for individuals coinfected with Trypanosoma cruzi and HIV since 2004. Although the first coinfection case was reported in the 1980s, its prevalence has not been firmly established. In order to know coinfection prevalence, a cross-sectional study of 200 HIV patients was performed between January and July 2013 in the city of Pelotas, in southern Rio Grande do Sul, an endemic area for Chagas disease. Ten subjects were found positive for T. cruzi infection by chemiluminescence microparticle immunoassay and indirect immunofluorescence. The survey showed 5% coinfection prevalence among HIV patients (95% CI: 2.0–8.0), which was 3.8 times as high as that estimated by the Ministry of Health of Brazil. Six individuals had a viral load higher than 100,000copies per μL, a statistically significant difference for T. cruzi presence. These findings highlight the importance of screening HIV patients from Chagas disease endemic areas.
American trypanosomiasis, also known as Chagas disease (CD), is a neglected tropical condition.1,2 The World Health Organization estimates that eight million people worldwide are presently infected with Trypanosoma cruzi.2
CD chronic infection is characterized by low parasite levels in the blood and in cardiac and/or digestive tract tissues, which typically persists throughout life. The chronic infection may manifest itself as indeterminate or symptomatic, and 20–30% of Chagas patients develop cardiomyopathy, megaesophagus, or megacolon.3 Nevertheless, the disease may seriously affect transplant recipients, cancer patients, and individuals living with AIDS due to immunosuppression.4,5 Indeed, T. cruzi, like other infectious organisms, is an opportunistic protozoan in these patients.6,7
Migration from rural to urban areas in Brazil and other Latin American countries has particularly increased the probability of individuals with Chagas disease to contract HIV.8,9 Consequently, Chagas disease reactivation in coinfected patients was declared an Aids-defining condition in 2004; as a consequence, the Brazilian Network of Care and Studies on T. cruzi/HIV coinfection was created in 2006.8,10,11 The 2008 Guidelines from the Brazilian Ministry of Health11 recommended a Chagas Disease serological test for all HIV patients, especially those from endemic areas, at the first medical assessment.
In those countries where CD is endemic, the coinfection HIV/T. cruzi rate ranges from 1.3% to 7.1%,12 whereas in Brazil the estimate is 1.3%.11 According to data from HIV/AIDS reports of the Ministry of Health in Brazil, the Southern and Central-Western regions of the country have the highest number of reported cases. Among municipalities with more than 100,000 inhabitants, the city of Pelotas occupies the twentieth position, with 5943 cases.9 In the same municipality, a study of 252 HIV+ patients13 measured the serologic testing index for Chagas, finding a 3.2% rate (eight patients), seven of whom were negative for trypanosomiasis and one had no results available in his medical record. The authors expressed concern on the low serologic testing index for CD in HIV+ patients, since the study was conducted in an area considered to be endemic for the presence of T. cruzi and its vectors.14,15
Given the lack of coinfection data in endemic areas and the relevance of the topic to public health, the aim of this study was to evaluate the T. cruzi/HIV coinfection prevalence in patients cared for at a specialized service center in the city of Pelotas, Rio Grande do Sul State, Brazil, as well as to evaluate coinfection correlation, if any, with gender, age, CD4+ T lymphocytes, and viral load.
A cross-sectional study was conducted with patients being monitored at in the Special Care Service (SCS) of the Medical School of the Federal University of Pelotas (UFPEL), Rio Grande do Sul State, Brazil. This service is a partnership with the Municipal Health Department of Pelotas, and provides care to public health system patients. The population under study comprised of 200 HIV infected patients, characterizing a representative SCS sample. The age of patients ranged between 18 to 80 years, and included both male and female patients. The study was performed between January and July 2013.
Socioeconomic, demographic, and behavioral information was collected according to a pre-tested structured questionnaire. The following data regarding socioeconomic and demographic variables were collected: residence in a T. cruzi endemic area (yes or no), gender (male or female), age group (up to 29, 30–39, 40–49, 50 years or older), education in school years (0–4, 5–8, 9 or more), marital status (married, single, widowed, or divorced), and monthly income (up to one or more than one minimum wage). The following behavioral variables were obtained: smoking, currently or up to the month before the interview (yes or no); alcohol intake currently or up to the month before the interview (less than once a week, more than once a week, every day, or never); current occasional drug use (yes or no). Treatment with antiretroviral therapy (yes or no), CD4+ T lymphocytes (up to 350 or >350cells/mm3), and viral load (<50, 51–100,000, or >100,000copies/μL) were obtained from medical records.
Blood samples were collected and tested for anti-T. cruzi IgG at the Clinical Analysis Laboratory of the Federal University of Pelotas. Samples were first tested by Chemiluminescent Microparticle Immunoassay (ARCHITECT Chagas®, Abbott). Positive results from this test were checked by indirect immunofluorescence (WAMA® Diagnóstica) according to manufacturer's instructions. Samples testing positive on both assays were considered infected, and test results were transferred to patient records and made available to both physicians and patients.
Sociodemographic, anti-T. cruzi IgG, and behavioral factors were analyzed by descriptive statistics using Stata® 12 (StataCorp LP, College Station, TX, USA). For analysis of coinfection against sociodemographic variables, CD4+ T lymphocytes, and viral load Fisher's exact test and logistic regression were used to compare proportions and obtain odds ratios, respectively.
The study was reviewed and approved by the Ethics Committee of the Medical School of the Federal University of Pelotas, Brazil according to Resolution 466/12 on research involving human subjects of the Brazilian National Health Council. All subjects of this research were adults and were asked to sign an informed consent after being informed on the purpose and procedures of the study.
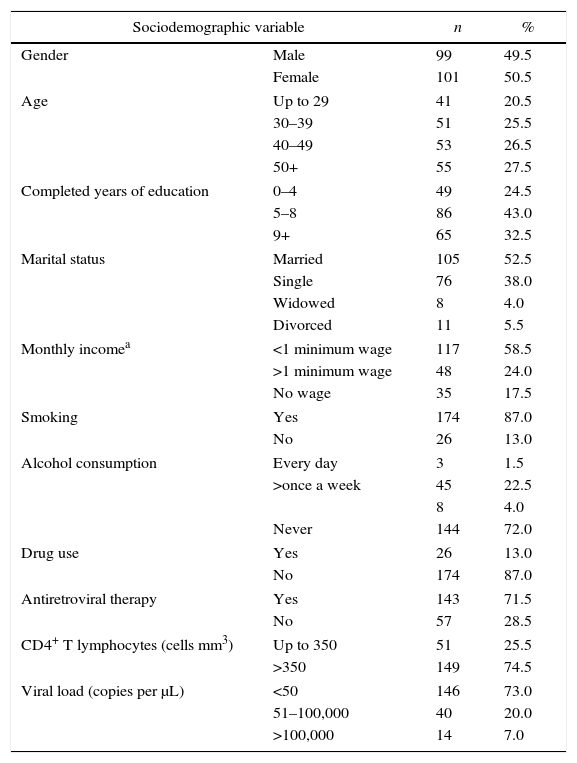
Table 1 shows sociodemographic and behavioral characteristics of the 200 patients who participated in the study. There were no refusals by respondents during the research. 49.5% (99) of the respondents were male and 50.5% (101), female. Most of the patients (54%) were 40 years of age or older, and 43% had 5–8 years of schooling, while 52% were married. Among those who reported having an income (82.5%), 58.5% earned up to one minimum wage. As to behavioral variables, 87% smoked, 28% had drunk alcohol in the previous month and 87% had never used illicit drugs. Most patients (71.5%) were undergoing antiretroviral treatment and 74.5% of the patients had LT CD4+ count higher than 350cells/mm3.
Sociodemographic and behavioral profile of patients surveyed for Trypanosoma cruzi/HIV coinfection in the extreme south of Brazil. n=200.
| Sociodemographic variable | n | % | |
|---|---|---|---|
| Gender | Male | 99 | 49.5 |
| Female | 101 | 50.5 | |
| Age | Up to 29 | 41 | 20.5 |
| 30–39 | 51 | 25.5 | |
| 40–49 | 53 | 26.5 | |
| 50+ | 55 | 27.5 | |
| Completed years of education | 0–4 | 49 | 24.5 |
| 5–8 | 86 | 43.0 | |
| 9+ | 65 | 32.5 | |
| Marital status | Married | 105 | 52.5 |
| Single | 76 | 38.0 | |
| Widowed | 8 | 4.0 | |
| Divorced | 11 | 5.5 | |
| Monthly incomea | <1 minimum wage | 117 | 58.5 |
| >1 minimum wage | 48 | 24.0 | |
| No wage | 35 | 17.5 | |
| Smoking | Yes | 174 | 87.0 |
| No | 26 | 13.0 | |
| Alcohol consumption | Every day | 3 | 1.5 |
| >once a week | 45 | 22.5 | |
| 8 | 4.0 | ||
| Never | 144 | 72.0 | |
| Drug use | Yes | 26 | 13.0 |
| No | 174 | 87.0 | |
| Antiretroviral therapy | Yes | 143 | 71.5 |
| No | 57 | 28.5 | |
| CD4+ T lymphocytes (cells mm3) | Up to 350 | 51 | 25.5 |
| >350 | 149 | 74.5 | |
| Viral load (copies per μL) | <50 | 146 | 73.0 |
| 51–100,000 | 40 | 20.0 | |
| >100,000 | 14 | 7.0 | |
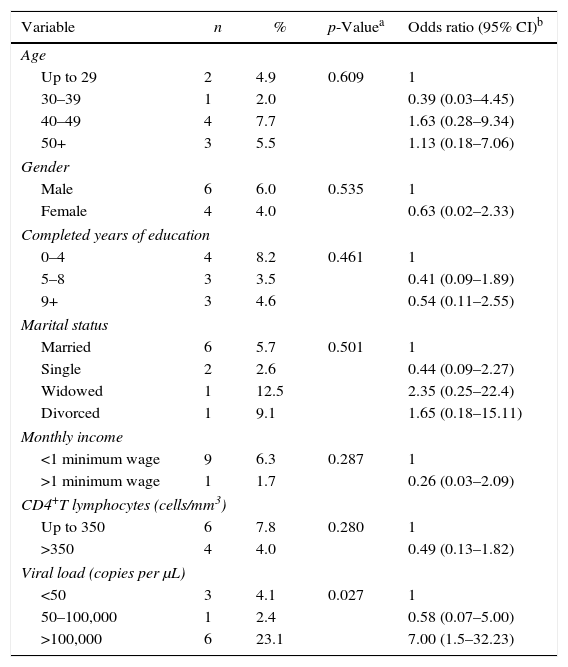
Ten individuals tested positive for T. cruzi, corresponding to 5% prevalence (95% CI: 2.0–8.0) among HIV patients. All were on antiretroviral therapy. The only variable significantly different between coinfected and monoinfected patients was the rate of viral load higher than 100,000copies per μL, as shown in Table 2.
Association of Trypanosoma cruzi/HIV coinfection in the extreme south of Brazil with sociodemographic factors, CD4+ T lymphocytes, and viral load. n=200, of which 10 were coinfected.
| Variable | n | % | p-Valuea | Odds ratio (95% CI)b |
|---|---|---|---|---|
| Age | ||||
| Up to 29 | 2 | 4.9 | 0.609 | 1 |
| 30–39 | 1 | 2.0 | 0.39 (0.03–4.45) | |
| 40–49 | 4 | 7.7 | 1.63 (0.28–9.34) | |
| 50+ | 3 | 5.5 | 1.13 (0.18–7.06) | |
| Gender | ||||
| Male | 6 | 6.0 | 0.535 | 1 |
| Female | 4 | 4.0 | 0.63 (0.02–2.33) | |
| Completed years of education | ||||
| 0–4 | 4 | 8.2 | 0.461 | 1 |
| 5–8 | 3 | 3.5 | 0.41 (0.09–1.89) | |
| 9+ | 3 | 4.6 | 0.54 (0.11–2.55) | |
| Marital status | ||||
| Married | 6 | 5.7 | 0.501 | 1 |
| Single | 2 | 2.6 | 0.44 (0.09–2.27) | |
| Widowed | 1 | 12.5 | 2.35 (0.25–22.4) | |
| Divorced | 1 | 9.1 | 1.65 (0.18–15.11) | |
| Monthly income | ||||
| <1 minimum wage | 9 | 6.3 | 0.287 | 1 |
| >1 minimum wage | 1 | 1.7 | 0.26 (0.03–2.09) | |
| CD4+T lymphocytes (cells/mm3) | ||||
| Up to 350 | 6 | 7.8 | 0.280 | 1 |
| >350 | 4 | 4.0 | 0.49 (0.13–1.82) | |
| Viral load (copies per μL) | ||||
| <50 | 3 | 4.1 | 0.027 | 1 |
| 50–100,000 | 1 | 2.4 | 0.58 (0.07–5.00) | |
| >100,000 | 6 | 23.1 | 7.00 (1.5–32.23) | |
Of the 200 individuals evaluated in this study, 10 were diagnosed with coinfection T. cruzi/HIV (5%), a rate 3.8-fold higher than the 1.3% estimate by the Ministry of Health in 2013.11 Thus, the survey highlights T. cruzi as a potential opportunistic parasite in HIV patients from areas where Chagas disease is endemic,16 such as southern Rio Grande do Sul, Brazil. A survey of HIV/T. cruzi coinfection in Europe in patients from Bolivia, Argentina, or the Southern Cone, confirmed a 1.9% coinfection.16 A study in Argentina, a country with the largest number of reported coinfection cases, along with Brazil,17 the prevalence of T. cruzi/HIV coinfection was 4.2%, similar to that in this study.18
High viral loads and a reduction in CD4+ T lymphocytes can lead to immunosuppression, and may be considered a reactivation risk factor,19 although there are no reliable methods of predicting this reactivation. In this study, most patients were on antiretroviral therapy, which appears to prevent or control Chagas reactivation.4 Indeed, the 10 coinfected individuals in this study had no symptoms consistent with Chagas reactivation. However, these patients need to be monitored carefully, as mortality may reach 80% if treatment is delayed for at least 30 days after the onset of Chagas symptoms, while early treatment reduces it to 20%.10
As to the variables analyzed, there was a statistically significant association only for coinfection and viral load above 100,000copies (OR=7.0). Although such association was found, one cannot be sure whether it is the T. cruzi parasite that caused this viral load increase. Nevertheless, evaluations have shown an association between CD reactivation, the decrease in CD4+ cell count, and increase in viral load.20 This association was not observed in this study, once CD reactivation cases were not detected. Therefore, other detailed reviews on this topic are needed.
This coinfection has been poorly characterized, and remains unknown to or neglected by many health professionals. Serological tests for Chagas disease in southern Brazil were requested at the first medical appointment for only 3.2% of HIV cases, even though the 2013 Consensus Document of the Ministry of Health recommends that such tests be requested for all HIV patients at the first appointment.11,13
Due to the possibility of the occurrence of both etiological agents in the same individual and the likely severity of this coinfection, it was concluded that the Ministry of Health guidelines as to the need for T. cruzi serological tests in HIV+ patients from CD endemic areas are relevant. Our study showed a coinfection rate 3.8-fold higher than that estimated for Brazil. Furthermore, patients who have been made aware of this condition can benefit from specialized medical care, thus avoiding eventual damage resulting from it.
FundingProvided by Programa de Apoio à Pós-Graduação (PROAP), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Brasília, DF, Brazil.
Conflicts of interestThe authors declare no conflicts of interest.
To SCS staff and the patients who participated in the survey.