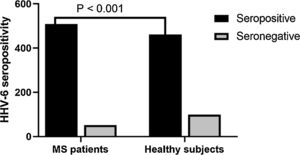
In recent years, extreme attention has been focused on the role of human herpesvirus-6 (HHV-6) in multiple sclerosis (MS) pathogenesis. However, the pathogenesis of MS associated with HHV-6 infection remains unknown. In this study, we measured the serum levels of matrix metalloproteinase-2 (MMP-2), matrix metalloproteinase-9 (MMP-9), and vitamin D levels in MS patients with HHV-6 infection and MS patients without HHV-6 infection. Five hundred sixty (including 300 females and 260 males) MS patients along with 560 healthy subjects were analyzed for HHV-6 seropositivity using enzyme-linked immunosorbent assay (ELISA). Subsequently, we measured the serum levels of MMP-2, MMP-9, and vitamin D levels in MS patients with HHV-6 infection and MS patients without HHV-6 infection by ELISA. About 90.7% of MS patients (508/560) were seropositive for HHV-6, while 82.3% (461/560) of healthy subjects were seropositive for this virus (p = 0.001). Moreover, there was a significant increase in the levels of MMP-2, MMP-9, and lower vitamin D in the serum samples of MS patients when compared with healthy subjects. Additionally, we demonstrated that the MMP-9 levels in seropositive MS patients were significantly higher than seronegative MS patients (p = 0.001). Finally, our results demonstrated that the mean of expanded disability status scale (EDSS) in seropositive MS patients was significantly higher in comparison to seronegative MS patients (p < 0.05). In conclusion, we suggest that the HHV-6 infection may play a role in MS pathogenesis.
MS is a neuroinflammatory disorder affecting the central nervous system (CNS), demolition of the myelin sheath, and axonal injury accompanying with diverging degrees of neurological impairment.1 Viral infection has long been a dilemma in the etiology of multiple sclerosis (MS) and related neuroinflammatory disorders. Epidemiological, pathological, and molecular investigations in the last decades have profoundly associated the human herpesvirus 6 (HHV-6) and human herpesviruses Epstein–Barr virus (EBV) with MS.2 HHV-6 is a neurotropic virus that frequently reactivates in immunocompromised patients.3 Two variants of HHV-6 have been recognized: HHV-6A, with a more prominent preference for infecting neural cells, and HHV-6B. However, it is less often associated with disease than HHV-6B.4 HHV-6A has been found predominantly in MS lesions. Primary evidence backing a convincing pathogenic role for HHV-6 in MS was based on cerebrospinal fluid (CSF) detection of viral DNA by polymerase chain reaction (PCR).4 Observation of viral messenger RNA and protein expression in oligodendrocytes were additional contribution to the hypothesis of HHV-6 as an operator of MS.5 Subsequent studies have revealed drastic variability in IgG- and IgM-specific responses to viruses in CSF, to viral antigens, as well as DNA detection from MS lesions.6–9
Furthermore, activation of HHV-6 and other herpes viruses (e.g., varicella-zoster, herpes simplex viruses, and herpesvirus-7 and 8) can trigger MS progression. Interestingly, marmosets (a small nonhuman primate) inoculated with HHV-6 developed an MS-like experimental neuroinflammatory condition when compared to controls.9,10 The role of vitamin D in MS etiology draws growing attention. The geographical distribution of the disease suggested the involvement of vitamin D in disease pathogenesis.11 Several studies have shown that vitamin D levels are lower in MS patients than in controls.12–16 Moreover, several studies of 25-hydroxyvitamin D levels after MS onset have noted correlations with MS risk, progression, disability, and severity.11 MMPs are a big family of zinc-containing endopeptidases that degenerate extracellular matrix and basement membrane composites (collagens, gelatin, laminin, fibronectin). Expression and activity of MMPs are tightly controlled.17 Among of all MMPs, MMP-9 and MMP-2 have been extensively investigated in MS given their capacity to degenerate components of the basal lamina and their involvement in blood-brain barrier (BBB) damage.18,19 Remarkably, increasing experimental evidence proposes the engagement of MMP-9 in the pathogenesis of MS, since the circulating levels of MMP-9 have been shown to be upregulated in MS patients, in contrast with other noninflammatory neurological disorders (NIND) and healthy subjects.20–23 To investigate the roles of MMP-9, MMP-2, and vitamin D in the pathogenesis of MS associated with HHV-6 infection, we determined serum MMP-9, MMP-2, vitamin D levels, and compared them among MS patients with or without HHV-6 infection. In addition, we assessed HHV-6 IgG titers in MS patients with HHV-6 infection.
Study designPatient and sample collectionFive hundred sixty (including 300 females and 260 males) patients clinically diagnosed with MS according to the diagnostic guidelines suggested by revised McDonald’ criteria,24 along with 560 healthy individuals (including 300 females and 260 males) were randomly chosen. The ethical committee of the Hamadan University of Medical Science approved (IR.UMSHA.REC.1398.409) the study. A trained neurologist determined the expanded disability status scale (EDSS) of patients. Inclusion criteria were: age 20–60 years, EDSS score less than 7.0 and written informed consent of all participants. Exclusion criteria were: to be on immunosuppressant drugs in the six months preceding study entry, and to have complications related to vitamin D deficiency, such as rickets or parathyroid diseases or use of drugs or supplements containing vitamin D or calcium. All MS patients had a relapse-remitting disease course and were on IFN-β treatment (for at least two years). Of note, blood samples were taken from all MS patients during the remission stage of the disease. In each study group, 5 mL of venous blood samples were drawn and then centrifuged to obtain a sufficient amount of serum. Sera were quickly frozen and stored at −70 °C until use. In this case-control study, we first investigated the frequency of HHV-6 infection in MS patients and healthy subjects (Table 1). After defining the status of HHV-6 infection, MS patients and healthy individuals were categorized into HHV-6 seropositive and seronegative groups (Table 2), and the levels of MMP-2, MMP-9, and vitamin D were evaluated in the serum of MS patients and healthy subjects.
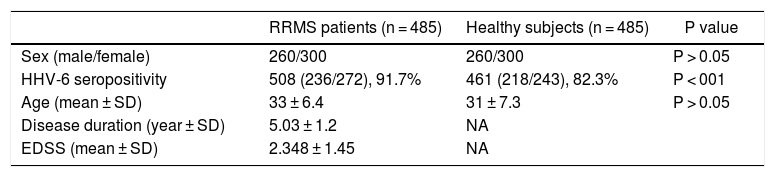
Demographic and clinical features of multiple sclerosis (MS) patients and healthy subjects.
| RRMS patients (n = 485) | Healthy subjects (n = 485) | P value | |
|---|---|---|---|
| Sex (male/female) | 260/300 | 260/300 | P > 0.05 |
| HHV-6 seropositivity | 508 (236/272), 91.7% | 461 (218/243), 82.3% | P < 001 |
| Age (mean ± SD) | 33 ± 6.4 | 31 ± 7.3 | P > 0.05 |
| Disease duration (year ± SD) | 5.03 ± 1.2 | NA | |
| EDSS (mean ± SD) | 2.348 ± 1.45 | NA |
RRMS, relapsing-remitting MS; SD, standard deviation; NA, not applicable; EDSS, expanded disability status scale.
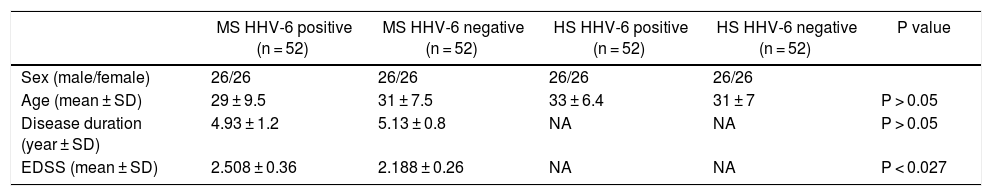
Four groups according to HHV-6 serostatus in multiple sclerosis (MS) patients and healthy subjects (HS).
| MS HHV-6 positive (n = 52) | MS HHV-6 negative (n = 52) | HS HHV-6 positive (n = 52) | HS HHV-6 negative (n = 52) | P value | |
|---|---|---|---|---|---|
| Sex (male/female) | 26/26 | 26/26 | 26/26 | 26/26 | |
| Age (mean ± SD) | 29 ± 9.5 | 31 ± 7.5 | 33 ± 6.4 | 31 ± 7 | P > 0.05 |
| Disease duration (year ± SD) | 4.93 ± 1.2 | 5.13 ± 0.8 | NA | NA | P > 0.05 |
| EDSS (mean ± SD) | 2.508 ± 0.36 | 2.188 ± 0.26 | NA | NA | P < 0.027 |
MS, multiple sclerosis; SD, standard deviation; NA, not applicable; EDSS, expanded disability status scale.
The anti-HHHV-6 IgG was measured in duplicate using a commercially available ELISA kit (HHV-6 IgG ELISA kit, Abnova, Taiwan) according to the manufacturer’s instructions. Optical density (OD) was measured at 450 nm using a BioRad 650 microplate reader (BioRad Laboratories Inc, USA). HHV-6 IgG index > 1.10 was considered as positive and less than < 0.90 negative. Intermediate index values between 0.90–1.10 were considered equivocal and excluded from the study.
Enzyme linked immunosorbent assay (ELISA) for MMP-2 and MMP-9The MMP-2 and MMP-9 were analyzed using a specific sandwich ELISA kit (MMP2 (ab100606) and MMP-9 (ab246539), Abcam Inc, Cambridge, MA, USA), according to the manufacturer’s instructions. In brief, 100 mL from each sample was added to each well. Then, secondary antibodies (biotinylated) and streptavidin labeled with horseradish peroxidase (HRP) was added to each well. A substrate solution tetramethylbenzidine (TMB) was applied for visualization. Optical density content was read at 450 nm using a BioRad 650 microplate reader. The presented data were the means of duplicate determinations.
Chemiluminescent immunometric assay for vitamin DThe serum levels of 25-hydroxyvitamin D were quantified using a quantitative chemiluminescent immunometric assay (DiaSorin, spA, Via Crescentino, Vercelli, Italy). The detection range was 4 ng/mL, and values under the limit of detection were computed as four ng/mL. Serum levels of 25-hydroxyvitamin D were classified into three groups: vitamin D deficient (under 20 ng/mL); vitamin D insufficient (21 through 29 ng/mL), and normal vitamin D levels (higher than 30 ng/mL).
Statistical analysisAnalysis of the collected data was performed using the SPSS software version 22. Quantitative variables are described as mean ± SD and qualitative variables as percentages. Differences between groups of variables with normal distribution were compared using Students T-test and one-way analysis of variance (ANOVA), followed by Tukey’s post-hoc test. In the case of non-normality, between groups differences were evaluated using the Kruskal-Wallis test, followed by Dunn's methods for pairwise checking. Graphs were depicted using the GraphPad software version 8. The level of statistical significance was set at p-value less than 0.05.
ResultsDemographic and clinical characteristicsAs shown in Table 1, all participants, including relapsing remitting multiple sclerosis (RRMS) patients and healthy individuals were age- and sex-matched. Age was not significantly differences between groups (p > 0.05), but there were more females in the control group (p-value less than 0.05).
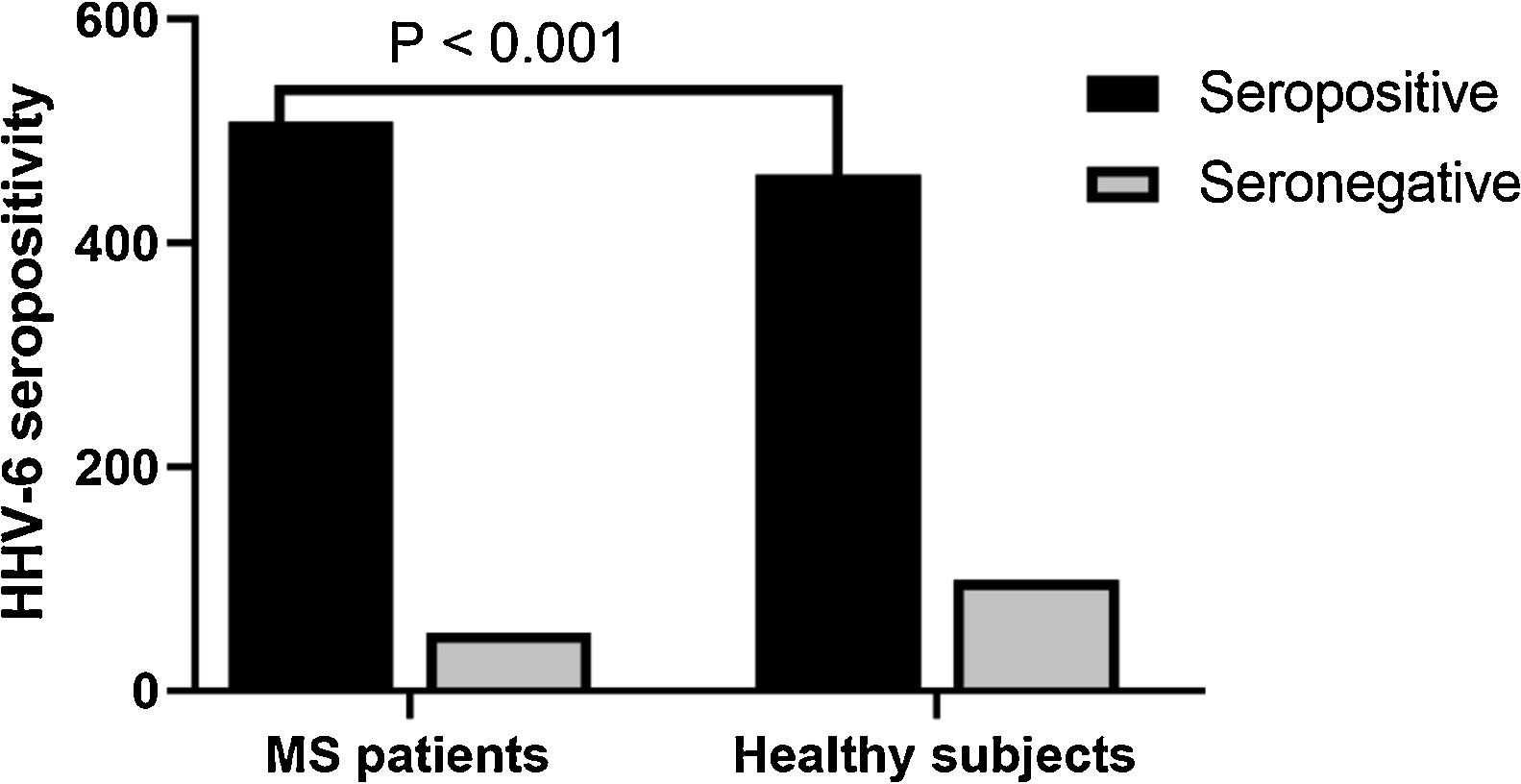
Anti-HHV-6 IgG in MS patients and healthy subjectsA total of 91.7% of MS patients were seropositive for HHV6 versus 82.3% of healthy subjects. (p < 0.001) (Fig. 1). After assessing HHV-6 serostatus, MS patients and healthy individuals were categorized into four groups; (1) seropositive MS patients; (2) seronegative MS patients; (3) seropositive healthy subjects; and (4) seronegative healthy subjects (Table 2). The mean EDSS of MS patients was 2.348 ± 1.45 and significantly higher in seropositive MS patients (2.508 ± 0.36) as compared to seronegative MS patients (2.188 ± 0.26) (p < 0.01).
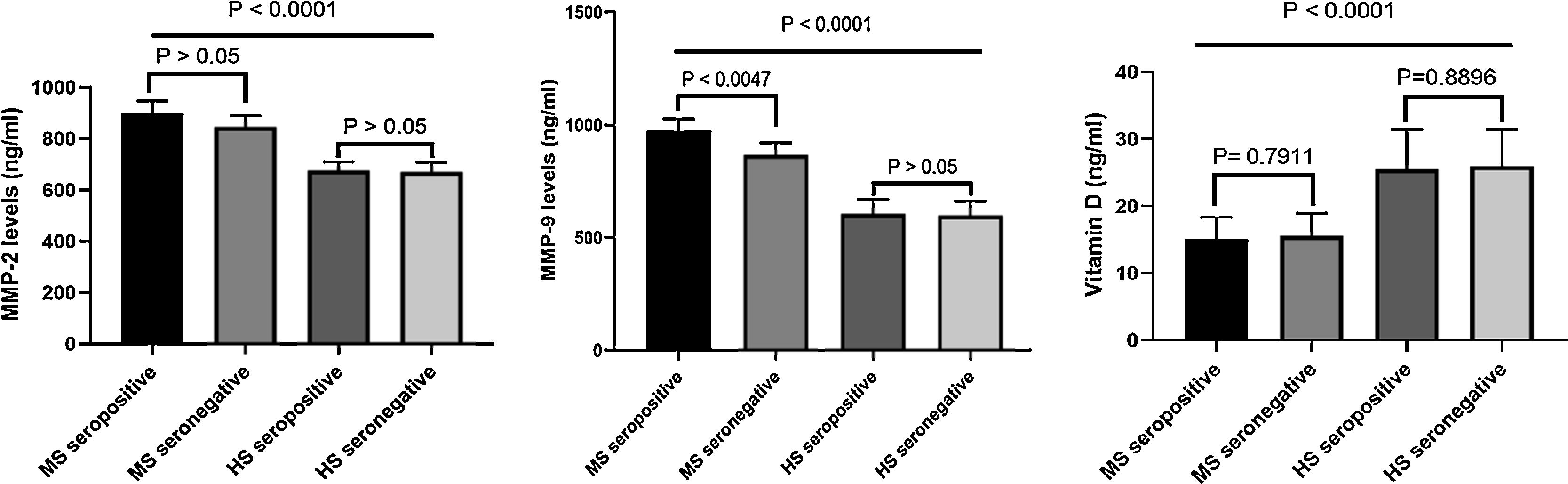
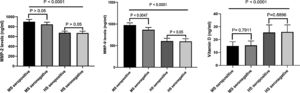
Levels of MMP-2 and MMP-9 in seropositive and seronegative MS patientsThe levels of MMP-2 were significantly higher in MS patients as compared to healthy subjects. Moreover, MMP-2 levels in seropositive MS patients was not different from seronegative MS patients (Fig. 2). In addition, serum levels of MMP-9 was higher in MS patients compared to healthy subjects. The MMP-9 levels were significantly higher in seropositive MS patients when compared to seronegative MS patients (p < 0.0047) (Fig. 2). No association was found between serum MMP-9 and MMP-2 levels with the EDSS (p > 0.05).
Chemiluminescent immunometric assaySerum of 25-hydroxyvitamin D levels in MS patients was significantly lower compared to healthy subjects (p < 0.0001) (Fig. 2). There was no significant difference in 25-hydroxyvitamin D levels among seropositive and seronegative MS patients (p > 0.7911). Besides, there was no association between vitamin D levels and EDSS.
DiscussionEnvironmental factors, such as viral and bacterial infections can accelerate, aggravate, or protect patients from MS.25 The vast majority of studies have suggested that HHV-6 is involved in the etiology of MS. Nevertheless, this association is controversial and, as is the case, experimental evidence linking this virus to MS is opaque.4,26 Pioneering studies describing HHV-6 viral DNA in the brains27,28 and CSF29 of MS patients and controls indicated that HHV-6 has strong neurotropism that was correlated with a CNS reservoir.27 Several clinical studies have proposed that MS and episodes of disease exacerbation are associated with concurrent viral or bacterial infections.30–32 However, there is still debate regarding the role of HHV-6 in MS pathogenesis. In this study, we demonstrated that the seropositivity of HHV-6 in MS patients was significantly higher than healthy subjects, as was observed in other studies.29,33–36 On the other hand, some studies have not shown significantly higher seropositivity rate of HHV-6 in MS patients.36–39 Besides, a number of studies found no correlation between MS and HHV-6.40–42 The hallmarks of MS are BBB disruption, migration of inflammatory cells to the CNS accompanying neuroinflammation, and neurodegeneration. Substantial experimental evidence suggests that MMPs play an active role in MS Experimental autoimmune encephalomyelitis pathogenesis.43 In vivo studies have revealed that MMP-2 null mice are far more susceptible to experimental autoimmune encephalomyelitis (EAE) because of the compensatory rise in MMP-9 in these animals.44 In EAE mice, increased levels of MMP-9 favors development and progression of the disease, while shifts in the levels of MMP-2 have a role in its resolution.45 These data proposed that the distinct MMPs enzymes might have distinguished roles in EAE development since the experiments have confirmed that the dual MMP-2 and MMP-9 knockout mice are resistant to EAE progression.46 Furthermore, we have demonstrated that serum levels of MMP-9 and MMP-2 in MS patients are significantly higher than in healthy controls. Furthermore, seropositive MS patients have significantly higher levels of MMP-9, but not MMP-2, than seronegative MS patients. Kittaka et al. reported a positive association between HHV-6 DNA and MMP-9/TIMP-1 levels in infants infected with HHV-6. These researchers stated that high serum levels of MMP-9 and TIMP-1 in infants infected with HHV-6 might influence the dysfunction of the BBB, ultimately inducing febrile seizures.47 Sanchooli et al. showed that the expression levels of CD154 isoforms, MMP-9, and MMP-2 in MS patients are significantly higher than in healthy subjects. Their results demonstrated that following an exacerbation period, sCD154 concentration increases, which is mutually related to the MMPs/TIMP-1 ratio.48 Moreover, our results demonstrated that there was no correlation between serum levels of MMP-2 and MMP-9 with EDSS in both seropositive and seronegative MS patients. The vast majority of observational research has proposed a relationship between vitamin D level in serum and MS risk, as well as disease activity.49 We have indicated that serum levels of vitamin D in MS patients are significantly lower than healthy subjects. Also, there were no significant differences in vitamin D levels among seropositive and seronegative MS patients. Besides, we observed no correlation between vitamin D levels and EDSS. Our results are in line with previous studies.16,50,51
All in all, our results illustrate that the HHV-6 seropositivity in MS patients was significantly higher than in healthy subjects. In addition, MMP-9 levels and EDSS in seropositive MS patients were markedly increased in relation to seronegative MS patients. However, the mechanism as to how HHV-6 could affect MS risk is uncertain, but several hypotheses have been suggested to explain how HHV-6 may play a causative role in MS development, including (1) direct cytopathic effect; (2) molecular mimicry; and (3) modulation of cytokine production during acute infection or virus reactivation, and an increase in an already active immune response during virus reactivation, a phenomenon also known as bystander effect. Finally, we suggest that HHV-6 infection may play a role in MS pathogenesis. Further work should be undertaken to further test the possible role of HHV-6 in MS pathogenesis.
FundingNone.
Ethical approvalThis study was approved by the ethical committee of the Hamadan University of Medical Science approved (IR.UMSHA.REC.1398.409).
Conflicts of interestThe authors declare no conflicts of interest.
We wholeheartedly thank Mr. Mirzaei and Mr. Shokri Moghadam for preparing the equipment to do the experiments. We also much appreciate the MS patients and healthy individuals take the time to participate in our study.