Plasmodium vivax and Plasmodium falciparum are becoming resistant to drugs including antifolates, sulphonamides and chloroquine. This study was focused at sequence analysis of resistant genes of these parasites against sulphadoxine–pyrimethamine and chloroquine, from Bannu, Pakistan. Known mutations were detected at codons 57, 58 and 117 of pvdhfr gene of P. vivax, while none of the isolates had any pvdhps mutation. Similarly P. falciparum isolates exhibited double 59R+108N mutations in pfdhfr, and single 437G in pfdhps thus demonstrating the existance of triple mutant 59R+108N+437G haplotype in this region. The key chloroquine resistance mutation, 76T in pfcrt was observed in 100% of the P. falciparum isolates, with haplotype SVMNT which is also associated with resistance to amodiaquine. Some novel mutations were also observed in pvdhfr and pfdhfr genes.
Plasmodium vivax and Plasmodium falciparum have been reported to develop resistance against drugs like sulphadoxine–pyrimethamine (SP) and chloroquine (CQ) in Pakistan.1 The development of multidrug resistance by P. falciparum and chloroquine resistance by P. vivax and insecticide-resistant mosquitoes has led to major difficulties in controlling the spread of malaria. Previous studies have suggested that resistance to SP is attributed to the emergence of certain mutations that occur sequentially in dihydropteroate synthase (dhps) and dihydrofolate reductase (dhfr) genes of Plasmodium.2,3 Rigorous work has been conducted to study the mutation structure of dhfr and dhps genes of P. falciparum but studies on P. vivax dhfr and dhps are limited to regions in Southeast Asia like Indonesia, Papua New Guinea, India and Sri Lanka.4,5
While PCR-RFLP methodologies have been developed for the detection of known polymorphisms, sequence analysis enables the detection of previously unknown mutations that may be playing some role in drug resistance.2 The sequencing approach is especially necessary for P. vivax, whose mechanisms of antimalarial drug resistance have not been clearly defined, although thought to be similar to those of P. falciparum.
This study is an extension of our previous study6 where drug resistance has been studied through PCR-RFLP. The objective was to know the overall gene sequence of P. falciparum and P. vivax isolates from Bannu district in Pakistan; a region with high prevalence of malaria, but where molecular studies of malaria parasites are particularly scarce.
As described earlier,6 two samples from patients infected with P. falciparum, 69 carrying P. vivax and 26 having mix infection were sequenced to analyze the frequency of drug resistance mutations. Sequence analyses was conducted at the Pfcrt, Pfdhfr, Pfdhps, PfmdrI, Pvdhfr, and Pvdhps genes using primers already published that allow to amplify fragments encompassing several codons in each gene.2,7–9 A total of 160 genes were cloned and sequenced consisting 10 Pfcrt, 23 Pfdhfr, 22 Pfdhps, 20 PfmdrI spanning codon 86 and 10 PfmdrI spanning codons 1042 and 1246; 40 Pvdhfr, and 35 Pvdhps. Each fragment was cloned using the TA cloning kit (Invitrogen) according to manufacturer's instructions. Bioedit and CLC DNA online softwares were used to investigate the phylogenetic relationships among the fragments of the genes analyzed.
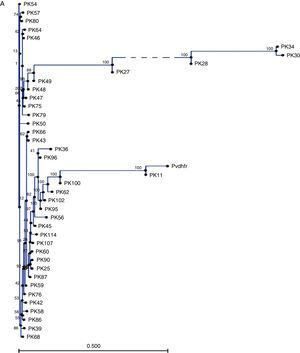
Table 1 summarizes the single nucleotide polymorphisms (SNPs) detected in the studied genes. 711bp of the pvdhfr gene sequence spanning codons 13, 33, 57, 58, 61, 117 and 173 were compared with the sequence of gene with a P. vivax isolate collected from Pakistan (GenBank accession no. X98123.1). Novel mutations were confirmed after sequencing at least two clones for each fragment. The majority of single nucleotide polymorphisms (SNPs) led to synonymous changes, with few exceptions (Table 1). A change from A to T at position 149 resulted in the substitution of a Q for an H in 12.5% of the samples. Similarly, two adjacent SNPs at positions 77 and 78 resulted in the emergence of a novel mutant type S93H. Similarly, we found a mutation of two nucleotides at positions 278 and 279 leading to S93H mutation in 16.5% of the P. vivax samples. However, the role of these mutations in conferring resistance against SP is still to be explored. Compared to the wild type reference, 17.5% of samples showed an insertion or deletion of an 18bp fragment (ACACACGGTGGTGACAAT) which was not taken into consideration during nucleotide count). Phylogenetic tree in Fig. 1A shows that 7 clusters of pvdhfr were obtained. Sample PK11 is closely related to the wild type strain shown as pvdhfr in the figure. However, it has less similarity with other isolates. 705bp region of pvdhps gene was sequenced and compared with wild type isolate from GenBank (accession no. AY186730.1). There was no change at codons 383 and 553.
Sequence analysis of portions of Pvdhfr, Pvdhps, Pfdhfr, Pfdhps, Pfcrt, PfmdrI and dhfr genes of P. vivax and P. falciparum parasites encompassing the regions with known drug resistant target-codons. P. vivax and P. falciparum parasites were obtained from blood samples of malaria patients from Bannu, Pakistan.
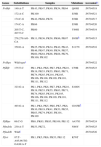
| Genes | Substitutions | Samples | Mutations | Accessiona |
|---|---|---|---|---|
| Pvdhfr | 149:A-T | PK43, PK47, PK49, PK54, PK64 | Q49H | JN794518 |
| 172:A-C | PK100 | S58R | JN794521 | |
| 174:C-G | PK42, PK68, PK76 | S58R | JN794517 | |
| 174:C-A | PK66 | S58R | JN794520 | |
| 205:T-C, 207:T-C | PK60 | Y69H | JN794519 | |
| 278,279:AG-CA | PK11, PK36, PK58, PK86, PK87 | S93Hb | JN794515 | |
| 350:G-A | PK25, PK30, PK34, PK42, PK43, PK46, PK47, PK49, PK54, PK57, PK60, PK64, PK66, PK68, PK79, PK100, PK102 | S117N | JN794516 | |
| Pvdhps | Wild typec | JN794522 | ||
| Pfdhfr | 175:T-C | PK1, PK4, PK6, PK7, PK8, PK10, PK19, PK53, PK55, PK63, PK71, PK74, PK85, PK101, PK105, PK106, PK108, PK109, PK110, PK111, PK112 | C59R | JN794523 |
| 323:G-A | PK1, PK4, PK6, PK7, PK8, PK10, PK19, PK53, PK55, PK63, PK71, PK74, PK85, PK101, PK105, PK106, PK108, PK109, PK110, PK111, PK112 | S108N | ||
| 493:G-A | PK1, PK4, PK5, PK6, PK7, PK8, PK10, PK19, PK41, PK53, PK55, PK63, PK74, PK85, PK101, PK109 | G165Rb | ||
| Pfdhps | 69:C-G | PK6, PK63, PK85, PK109, PK112 | A437G | JN794524 |
| PfmdrIa | 230:A-T | PK53, PK72, | N86Y | JN794525 |
| PfmdrIb | Wildc | JN794526 | ||
| Pfcrt | 87:T-A95,96:TG-GA98:A-T | PK1, PK5, PK6, PK53, PK112 | K76T | |
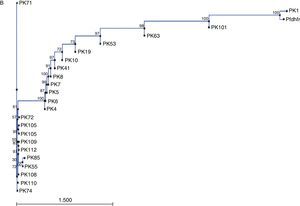
Sequences of Pfdhfr gene spanning codons 16, 50, 51, 59, 108 and 164 were compared with GenBank sequence (accession no. J04643.1). Sequence homology among these isolates is represented by the neighbor joining phylogenetic tree in Fig. 1B. Sequence variation at pfdhps locus was determined by comparing sequence data of samples with wild type isolate from GenBank (accession no. Z30659.1). In 31.8% of the isolates, a SNP at position 69 led to the mutation A437G. A 145bp region of the pfcrt gene was compared with GenBank wild type isolate (accession no. AF030694.2) and it was found that all the samples carried SVMNT haplotype. The sequence data of the pfmdrI gene were compared with wild type sequence from GenBank (accession no. X56851.1). All samples carrying any non-synonymous mutation and a representative wild type sequences were submitted to the GenBank (Table 1).
This study was aimed at sequencing the entire genes involved in drug resistance to find out the involvement of any possible new mutations in conferring drug resistance. Though it has been found earlier that SP drug resistance is prevalent in Bannu and PCR-RFLP showed some known mutations,6 to have the genetic picture of the organisms it was important to sequence the entire genes. So our aim was to identify the level of polymorphism and possibly new mutations that need to be studied further in order to assess their implication on clinical efficacy of SP. SP is effective in patients having P. vivax infection with wild-type genotype or even a 58R-117N double mutant allele10 so other mutations are clearly involved. Sequence analysis showed polymorphism in this gene and some unique mutations like Q49H, Y69H and S93H have also been found but their role in conferring drug resistance against SP is still to be elucidated.
No pvdhps mutation was detected in isolates from our study area. Therefore, it can be said that Bannu is an area where SP pressure in low, similar to earlier findings.9
Mutations in pfdhfr gene are said to emerge in a stepwise manner4 with S108N emerging initially followed by N51I or C59R and lastly I64L mutant type. By comparison we observed mutations at codons 59 and 108 in all our isolates but no mutation was observed at codons 51. McCollum and colleagues while working with African isolates opposed this theory by finding 51I/108N/164L genotype.11 We did not find A16V and S108T mutations that are thought to be responsible for resistance against the drug Cycloguanil which again support the findings of Garg and colleagues.12 A non-synonymous mutation G165R was detected in pfdhfr gene which to our knowledge is a novel mutation.
The majority of isolates were found to contain wild-type pfdhps gene.
We detected haplotype 86Y-1042Y, in Pfmdr1 gene unlike in West Africa where 86Y-86N-1246D haplotype occurred more commonly.13 Mayengue and colleagues while working with isolates from Gabonese patients found Pfmdr1 86Y and 184F mutations in their samples which are in accordance with our findings though we did not analyze mutations at codon 184.14
We found haplotype SVMNT in all our isolates which is also found in isolates from Papua New Guinea and South America. The SVMNT haplotype of pfcrt is also considered to be linked with chloroquine resistance, and, like the K76T mutation, it is common among Indian field isolates15 which is in accordance with our findings. CVIET haplotype is more prevalent in Asia and Africa while SVMNT is associated with Papua New Guinea and South American isolates16 while SVIET, CVIKT, CVIDT and CVTNT have been found in Southeast Asia.17 High level of mutation in pfcrt gene shows that P. falciparum has developed resistance against CQ. It could be due to the fact that areas like Pakistan where mixed infections by both P. vivax and P. falciparum exist and CQ is prescribed for treatment of P. vivax malaria thus inadvertently putting P. falciparum under selective drug pressure which leads to emergence of CQ resistance, as observed in the Mekong Delta.18
In this study, sequence analysis of key genes associated with antimalarial drug resistance showed that P. falciparum and P. vivax in the Bannu district of Pakistan have not yet reached an alarming threshold against drugs like sulfadoxine–pyrimethamine unlike in many parts of the world but resistance seems to be on the rise. However, P. falciparum seem to have already acquired considerable resistance against CQ. Moreover, some novel mutations, whose roles in malaria drug resistance are unknown, were identified.
Conflict of interestThe authors declare no conflict of interest.
We thank Higher Education Commission of Pakistan for financial support of this research work for PhD of Ms. Lubna Khatoon at Quaid-i-Azam University Islamabad Pakistan and University of California, Irvine, USA.