Patients with hepatitis C virus-related decompensated cirrhosis can benefit from interferon-based antiviral therapy, but the common complication of cytopenia is a contraindication for this treatment. Splenectomy prior to interferon therapy may alleviate this problem. To investigate whether splenectomy improves the efficacy of antiviral therapy, 13 interferon-naïve hepatitis C virus decompensated cirrhotic patients underwent splenectomy between January 2008 and January 2011, followed 1–3 months later by an interferon-based therapeutic regimen (pegylated/standard interferon-α combined with ribavirin for 48 weeks). Ten (76.9%) of the patients developed postoperative complications, which included minor portal vein thrombosis (2/13, 15.4%) and transient ascites (8/13, 61.5%). At one-month post-splenectomy, the patients showed significantly increased platelet (pre-surgery: 48.2±15.9 vs. 186.0±70.6×103μL−1, p<0.001) and leukocyte (2.1±0.5 vs. 5.7±1.4×103μL−1, p<0.001) counts. Eight (61.5%) of the patients achieved sustained virological response, including all HCV genotype 2a-infected patients (4/4, 100%) and some of the genotype 1b-infected patients (4/9, 44.4%). Temporary interferon-α suspension was required for one patient to address severe intestinal infection. These results indicate that splenectomy prior to interferon-based therapy was safe and may facilitate adherence to subsequent antiviral therapy in selected HCV cirrhotic patients with portal hypertension and hypersplenism.
Hepatitis C virus (HCV) is a major public health problem. Approximately 170 million people are currently infected worldwide. Chronic infection is frequent and is strongly associated with development of cirrhosis within ∼20 years of the original diagnosis.1 Improvements in antiviral drugs, such as the pegylated (PEG) form of interferon (IFN), and treatment regimens, such as the IFN-based combination therapy with ribavirin, have achieved higher rates of sustained virological response (SVR) and improved long-term prognosis.2,3 However, not all chronic hepatitis C patients are amenable to the IFN-based therapies and some develop contraindications that necessitate treatment suspension, reduced dosage, or termination of therapy.4,5 When the threat of unmitigated chronic hepatitis C cases was investigated in a mathematical model of the disease's natural progression, researchers predicted a dramatic increase in the number of HCV-related cirrhotic cases over the next two decades.6
Liver cirrhosis is the manifestation of structural changes in the liver tissue, such as fibrotic deposition and scarring, which occur as a result of long-term liver disease. Once the structural damage comprises ∼80% of the organ, the liver cirrhosis progresses to the decompensated stage when the liver is no longer able to function properly. Decompensated cirrhosis patients have a very high frequency of hospital admission, a generally low quality of life, and high risk of mortality.4 Liver transplantation remains the only successful therapeutic option for these patients, but the limited number of organ donors and high expense severely restricts its applicability.
Investigators aiming to identify feasible and efficacious therapies for patients with HCV-related compensated and decompensated cirrhosis have observed beneficial results from IFN-α-based antiviral therapy.3,4,7 Unfortunately, a large proportion of these patients have preexisting neutropenia, thrombocytopenia, and anemia that tend to worsen with the use of IFN and ribavirin and necessitate dose reduction or treatment discontinuation.4,5 In addition, some patients present with severe baseline thrombocytopenia and leukocytopenia due to hypersplenism that preclude initiation of IFN-based therapy. New non-IFN therapeutic approaches using combinations of direct-acting antiviral agents (DAAs) with or without ribavirin have been developed. While the early studies of these DAA-based approaches have shown promising results for rapid and profound inhibition of HCV RNA, the efficacy and safety of such regimens have yet to be established, especially in complicated cases.8
Another therapeutic strategy that may overcome the limitations of cirrhosis-related complications and improve the efficacy of antiviral therapies is pre-emptive splenectomy.9,10 However, this alternative treatment remains largely theoretical and its appropriateness is controversial. This clinical study was designed to prospectively investigate the safety and efficacy of prior splenectomy for improving antiviral therapeutic effects in decompensated cirrhosis patients with HCV infection.
This study was approved by the Institutional Ethics Committee of the Second Affiliated Hospital of the College of Medicine at Xi’an Jiaotong University (March 20, 2008). Between January 2008 and January 2011, patients diagnosed with HCV-related decompensated cirrhosis were recruited to the study according to the following eligibility criteria: no prior antiviral therapeutic intervention; adequate liver function (Child-Pugh class A or B); thrombocytopenia (platelet count: <80×103μL−1); severe esophagogastric varices with or without variceal bleeding; no severe comorbidities, such as hepatocellular carcinoma or chronic renal failure. The diagnosis of decompensated liver cirrhosis was made when a patient had experienced one or more of the following clinical symptoms: ascites, variceal bleeding, spontaneous bacterial peritonitis (SBP), and encephalopathy. Of the 1092 consecutively admitted cirrhotics, 198 were excluded for hepatocellular carcinoma, 710 for non-HCV-related disease (586 HBV, 65 alcoholic, 58 biliary cirrhosis and one patients with haemochromatosis), 37 for inadequate liver function (Child-Pugh class C), 28 for chronic invalidating disease (such as unstable cardiovascular disease, chronic renal failure, or severe chronic obstructive lung disease), 23 for being older than 65 years, 5 for previous combination antiviral therapy, 28 for refusing to be treated, and 50 patients with moderate hypersplenism and portal hypertension received pegylated/standard IFN-α plus ribavirin combination therapy with a low accelerating dosage regimen for 48 weeks (partial results has been described in previous study5). The remaining 13 patients were enrolled into the study. The characteristics of the 13 patients are presented in Table 1. All the patients were clearly informed about the benefits and risks of the therapy. Written, signed consent was obtained from all participants. The study was performed without any financial support from producers of drugs.
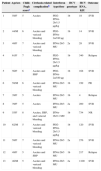
Clinical characteristics and therapeutic outcomes of treated patients.
| Patient | Age/sex | Child-Pugh scorea | Cirrhosis-related complicationb | Interferon type/dose | HCV genotype | HCV RNA, KIU | Outcome |
|---|---|---|---|---|---|---|---|
| 1 | 58/F | 5 | Ascites | PEG-IFNα-2b/1.5μg/kg | 1b | 18 | SVR |
| 2 | 44/M | 6 | Ascites and variceal bleeding | PEG-IFNα-2a/180μg | 1b | 14 | SVR |
| 3 | 48/F | 7 | Ascites and variceal bleeding | IFNα-2b/3 MU | 2a | 28 | SVR |
| 4 | 61/F | 7 | Ascites | PEG-IFNα-2b/1.5μg/kg | 1b | 340 | Relapse |
| 5 | 56/F | 6 | Ascites and SBP | PEG-IFNα-2a/180μg | 1b | 168 | SVR |
| 6 | 58/M | 6 | Ascites and variceal bleeding | IFNα-2b/3 MU | 1b | 199 | PR |
| 7 | 39/F | 5 | Ascites | IFNα-2b/3 MU | 1b | 4 | Relapse |
| 8 | 39/F | 5 | Ascites | IFNα-2b/3 MU | 2a | 200 | SVR |
| 9 | 55/F | 8 | Ascites, SBP, and variceal bleeding | IFNα-2b/2.1 MU | 1b | 734 | NR |
| 10 | 62/M | 5 | Ascites and variceal bleeding | PEG-IFNα-2b/1.5μg/kg | 1b | 120 | SVR |
| 11 | 58/F | 7 | Ascites and variceal bleeding | IFNα-2b/3 MU | 2a | 276 | SVR |
| 12 | 49/F | 5 | Ascites and SBP | IFNα-2b/3 MU | 1b | 217 | Relapse |
| 13 | 46/M | 5 | Ascites and variceal bleeding | IFNα-2b/3 MU | 2a | 1100 | SVR |
PEG-IFN-α, pegylated-interferon-alpha; IFN-α, interferon alpha; SBP, spontaneous bacterial peritonitis; SVR, sustained virological response; PR, partial response; NR, null response.
Open splenectomy with azygoportal disconnection surgical procedure was performed as described previously.11 Peripheral blood samples were collected immediately prior to surgery and on post-surgery days 1, 3, 7, 10 and one-month for routine monitoring of patient status and analysis of biomarkers of HCV infection and liver function. When platelet counts exceeded 300,000/μL, aspirin was administered. Abdominal ultrasound was performed between post-surgery days 7 and 10 to assess portal vein thrombosis (PVT). When PVT occurred, warfarin was administered.
For all patients, antiviral treatment was initiated at one to three months after splenectomy. The 48-week combination therapy regimen was assigned according to the HCV genotype. Patients infected with HCV genotype 2a were treated with the combination of standard (non-pegylated) IFNα-2b (3 MU subcutaneous injections, thrice weekly) plus ribavirin (800mg orally, daily). For nine patients infected with HCV genotype 1b, two patients received PEG IFNα-2a (180μg subcutaneous injections, once weekly), three patients received PEG IFNα-2b (1.5μg/kg subcutaneous injections, once weekly) and the other four patients received standard IFNα-2b (3 MU subcutaneous injections, thrice weekly) in combination with ribavirin (800mg orally for patients weighing<60kg and 1000mg orally for patients weighing>60kg, daily) for 48 weeks. Patients were monitored for drug-related complications, such as cytopenia, to facilitate timely dose reductions or discontinuation of antiviral treatment. Patients were routinely monitored for SVR, relapse, partial and null response according to the accepted guidelines.12
All data values are expressed as mean±standard deviation. Statistical comparisons between pre- and post-operative data were made using paired t-test. A p-value of <0.05 was considered statistically significant.
Of the 13 patients who received splenectomy with azygoportal disconnection in this study, four had previously underwent abdominal surgery, including cholecystectomy (n=2), uterine-incision delivery (n=1), and enterectomy (n=1). The mean surgical duration of the splenectomy procedure was 193±27.5min (range: 160–260min), and the mean blood loss was 163±65mL (range: 100–340mL). All patients survived and none experienced infectious complications. Minor PVT was detected by ultrasound in two patients, and eight patients developed transient ascites. Histologic evaluation of the resected spleen specimens showed congestive splenomegaly in all 13 patients.
As shown in Table 2, some of the liver function markers were improved at one-month after splenectomy. The Child-Pugh class improved for 5 patients but remained unchanged for the remaining 8 patients. The pooled average Child-Pugh class score at one-month was not significantly different from baseline. However, serum alanine transaminase levels were moderately elevated in the majority of patients, and the mean level at one-month was significantly higher than that at baseline. Significant increases were also observed in mean platelet count (one-month post-splenectomy: 186.0±70.62×103μL−1 vs. baseline: 48.23±15.92×103μL−1, p<0.001), mean leukocyte count (5.695±1.423 vs. 2.102±0.492×103μL−1, p<0.001) and mean neutrophil count (2.045±0.674 vs. 0.977±0.203×103μL−1, p<0.001).
Liver function markers before and one month after splenectomy (n=13).
| Marker | Before | After (one month later) | p-Value |
|---|---|---|---|
| STB, μmol/L | 22.83±8.60 | 18.75±9.06 | 0.115 |
| ALT, IU/L | 57.77±41.52 | 91.23±52.64 | 0.006 |
| Serum albumin, g/dL | 36.84±2.49 | 37.30±4.68 | 0.669 |
| PTA, % | 71.55±10.44 | 78.41±10.25 | 0.035 |
| Mild ascites, n | 2 | 0 | NA |
| Encephalopathy, n | 0 | 0 | NA |
| CPS | 6.00±1.00 | 5.85±0.69 | 0.337 |
STB, serum total bilirubin; ALT, alanine transaminase; PTA, prothrombin time activity; CPS, Child-Pugh score; NA, not applicable.
All 13 patients completed 48 weeks of antiviral therapy and at least 24 weeks of follow-up. However, one case (patient 5#) required a 2-week suspension after 23 weeks of combination therapy with PEG-IFNα-2a and ribavirin while a severe intestinal infection was treated, and three other patients required a dosage reduction of standard IFNα-2b (patient 9#) and ribavirin (patient 4# and 8#). None of the patients withdrew from the study due to cytopenia or any other complications during antiviral treatment. SVR was achieved in all four patients infected with genotype 2a (100%) and in four of the nine patients infected with genotype 1b (44%). Among the remaining five patients who did not achieve SVR, three relapsed, one showed only a partial response, and one showed a null response. Therapeutic outcomes of all 13 patients are presented in Table 1. No patients experienced variceal bleeding or encephalopathy during antiviral treatment or during the median 19 (range 7–32) months of follow-up off-therapy. There was no death or a need for liver transplant, although patient 9# and patient 4# experienced new episode of SBP and ascites in 9 and 18 month after end-of-treatment, respectively.
Patients with HCV-related decompensated cirrhosis are associated with poor prognosis. This difficult-to-treat population was recently identified as appropriate for IFN-α-based antiviral therapy.4,7,12 However, the common complication of severe thrombocytopenia has proven to be a contraindication for IFN and ribavirin drugs. In a preliminary prospective study, we had evaluated the efficacy and safety of standard IFN-α/ribavirin combination therapy with a low accelerating dosage regimen for HCV-related decompensated cirrhosis.5 Even though by the end of treatment 66.7% of the examined patients had undetectable level of HCV RNA and improved liver function, 50% of those patients eventually relapsed.5 We speculated that this high relapse rate could be related to inadequate dose and/or therapy duration. In addition, 25% of those patients experienced severe adverse events, including severe thrombocytopenia with extensive hemorrhage, SBP, and variceal bleeding,5 which is a major cause of death in cirrhotic patients. Considering these results, we speculated that a pre-emptive treatment strategy, to overcome the initial complications, might help improving the efficacy of subsequent antiviral therapy. Thus, we designed the current study to investigate the efficacy and safety of open splenectomy and azygoportal disconnection to reverse thrombocytopenia and improve subsequent antiviral therapy in selected patients with HCV-related decompensated cirrhosis.
The current study, which included nine patients classified as Child-Pugh grade A and four patients classified as Child-Pugh grade B, indicated that this pre-emptive strategy was safe. Only two patients developed minor PVT, which was completely resolved in one patient by a two-week course of warfarin. All patients showed improved or unchanged liver function by one month after surgery, which is remarkable considering that eight patients experienced transient ascites. These findings agree with previous reports of splenectomized patients showing a small decrease in the mean model of end-stage liver disease (MELD) score and/or Child-Pugh score following surgery.9–11 In the current study, splenectomized patients also showed a normalized level of platelet and leukocyte counts that was sustained for one-month after surgery, thereby allowing these patients to undergo subsequent antiviral therapy without hematologic disturbance. All 13 study participants underwent full-dose pegylated or standard IFN-α therapy with ribavirin, which was initiated within one to three months after the splenectomy surgery. Only one of those patients required transient IFN-α interruption to address a severe intestinal infection and a few others required reduced antiviral dosages. Importantly, no patient required premature treatment withdrawal and all patients completed the 48-week course without developing variceal bleeding or encephalopathy and while remaining complication-free during the 24-week follow-up.
Another intriguing finding from the current study was that 100% (4/4) of HCV genotype 2-infected patients achieved SVR while less than 50% (4/9) of HCV genotype 1-infected patients achieved SVR. Although the number of eligible cases examined was exceedingly small, the antiviral treatment results are similar to those previously reported for larger cohorts of patients without thrombocytopenia,13,14 suggesting that splenectomy does not reduce the antiviral efficacy of IFN-based treatment.
The pre-emptive strategy of splenectomy for patients with HCV-related cirrhosis to facilitate antiviral therapy has not been practiced widely. Very few studies to date have provided evidence that splenectomy would be conducive to eradicate the virus in addition to improving thrombocytopenia and leukocytopenia.15,16 In one study by Sekiguchi et al., splenectomized HCV-related cirrhotic patients were shown to experience decreased virus burden through augmentation of their natural killer cell activity.15 In another study by Hashimoto et al., the splenectomy procedure was demonstrated to facilitate viral clearance by inducing recovery of IFN-γ production and peripheral CD4+ T cell proliferation.16 Certainly, any effect on the immune system that are induced upon splenectomy could help establishing a beneficial milieu for the antiviral mechanisms of IFN-α and ribavirin. For example, peripheral tolerance is partially promoted by splenic-induced up-regulation of programmed death-1 (PD-1) ligands, and the fact that splenectomy is followed by a reduction in PD-1-expressing CD4+ T cells in peripheral blood may explain the enhanced eradication of HCV.16
Another important aspect of the current study is the Asian ancestry of the patient population. Asians have a significantly higher probability of viral response than Caucasians and Africans,17,18 and this character has been attributed to genetic variants in the interleukin (IL)-28B gene.18,19 None of our patients underwent IL-28B genotyping, and future studies of pre-emptive splenectomy should assess the potential contribution of this genotype to the SVR rates achieved. It is possible that a particular IL-28B variant may identify a population of patients that are likely to benefit most from this pre-emptive surgical strategy, so that genotyping may be a useful method to avoid the burden and cost of unnecessary surgery.
Recently, a triple combination therapy of first-generation protease inhibitors plus PEG-IFN and ribavirin has been administered to compensated cirrhosis patients with HCV genotype 1 infection.20 However, its feasibility for treating patients who have reached the decompensated stage remains unknown. Preliminary results with the protease inhibitors telaprevir or boceprevir have been promising, which leads us to speculate that such a broader range combination therapy regimen may further benefit the pre-emptive splenectomy strategy for treating various cirrhotic patients with genotype 1 and high viral loads.
There are some limitations in our study: small sample size, observational study, no standard-of-care antiviral treatment in all patients, not compared to patients without treatment, short follow up, etc. Despite these limitations, several conclusions can be drawn based upon careful consideration of the available data.
Splenectomy and azygoportal disconnection may represent a safe and effective strategy for improving thrombocytopenia, reducing the risk of esophagogastric varices bleeding, and promoting efficacy of subsequent antiviral therapy in selected HCV-related cirrhosis patients with portal hypertension and hypersplenism. Further investigations with larger cohorts of patients are required to confirm the feasibility of this procedure in clinical application and uncover its underlying mechanisms.
Conflict of interestThe authors declare no conflict of interest.
This work was supported by National Natural Science Foundation of China (No. 30901268) and The Health Research Foundation of Shaanxi Province (No. 2010H31). We are grateful to Dr. Hongan Xue, Zhifang Cai, Layang Liu, An Jiang, and Zhidong Wang for their participation in partial work of this study.