Chronic kidney disease (CKD) patients undergoing hemodialysis (HD) are more vulnerable to blood-borne viral infections due to frequent invasive procedures. Hepatitis B virus (HBV) infection in this cohort of patients has been a matter of concern worldwide. The objective of this cross-sectional study was to evaluate the frequency of serological markers for hepatitis B, and the occurrence of overt and occult HBV infection (OBI) and its molecular characterization in serum samples from 644 CKD patients in HD units located in Rio de Janeiro, Brazil, from 2013 to 2017. HBV DNA was investigated in HBsAg reactive and “anti-HBc alone” samples to determine infecting genotypes and genetic relatedness between sequences. The prevalence of serological markers HBsAg+, anti-HBc alone, anti-HBc+/anti-HBs+, anti-HBs+, anti-HBc/anti-HBs/HBsAg were 5.9%, 2.8%, 30.7%, 26.6%, 34.0%, respectively. HBV DNA was detected in 39.5% (15/38) of the HBsAg+ and in 5/18 (27.8%) of the “anti-HBc alone” individuals, indicating a high prevalence of OBI within this group. We found a higher prevalence of HBV/A1 (65%), followed by HBV/D3 (20%), and HBV/A2 (15%). Bayesian MCC tree with a highly supported clade, genetic distance comparison, and identical nucleotide sequences suggested a nosocomial spread of HBV in some units. The high prevalence of HBV infection and low number of individuals immune to infection reinforces the need for vaccination in this group. The presence of closely related strains in the same HD unit reinforces the importance of continuous improvement of safety control measures and laboratory surveillance of serological markers to prevent the risk of infection and transmission of HBV.
Chronic kidney disease (CKD) is characterized by the permanent loss of kidney function and is considered a global public health issue, affecting more than 133,000 people in Brazil.1,2 According to the Brazilian Chronic Dialysis Survey in July 2017, a yearly increase in the number of patients on dialysis was noted and nearly half of them were concentrated in the southeastern region.2 One of the major concerns regarding dialysis treatment is the dissemination of blood-borne infectious diseases, like hepatitis B, which is considered the most common viral infection transmitted by this procedure.3
Hepatitis B virus (HBV) is an etiologic agent of human liver diseases, including acute and chronic hepatitis, cirrhosis, and hepatocellular carcinoma.4 It is considered a major global health problem with an estimated 260 million people chronically infected worldwide.5 The identification of HBV surface antigen (HBsAg) by serological methods is the main diagnostic tool for accessing acute or chronic HBV infection. Improvements in the sensitivity of molecular biology techniques seen in the last decades however, have allowed the detection of low HBV DNA levels in serum samples and/or livers of individuals negative for HBsAg, a condition known as occult hepatitis B infection (OBI).6,7
The characterization of different HBV genotypes in certain populations is epidemiologically useful in tracking patterns of HBV transmission and the introduction of ‘foreign’/imported strains.8 In Brazil, HBV genotype A is the most prevalent, followed by genotypes D and F. Genotypes E, G, C and B are found occasionally.9
Despite being a vaccine-preventable disease, several cases of nosocomial transmission have been reported over the years even in high-income countries and, especially for hemodialysis units, are often related to flaws in standard precautions of cleaning and disinfecting equipment and instruments.10,11 Previous Brazilian studies have reported a prevalence rate of positive serology for hepatitis B varying from 0.8% to 15.4% in different hemodialysis units.2,12-14 Tracking of OBI cases in patients undergoing dialysis is particularly important since this condition is not detected by HBV serological markers while infected individuals are potential sources of viral dissemination.
The aim of this study was to determine the prevalence of serological markers for HBV infection in individuals with chronic kidney disease attending hemodialysis units in Rio de Janeiro, Brazil, monitoring the occurrence of occult HBV infection and indicating the genotypic distribution of HBV among positive cases.
Materials and methodsStudy populationSerum samples were collected from 644 individuals with chronic kidney disease treated at three private hemodialysis clinics located in the State of Rio de Janeiro between September 2013 and February 2017. The three hemodialysis units were located in the cities of Rio de Janeiro (n = 355), Niterói (n = 117), and Queimados (n = 172). Individuals or legal guardians who agreed to sign the informed consent form were included in the study. All participants were aged over 14 years old and in active dialysis treatment due to chronic kidney disease. Demographic data were collected from the patients’ medical records and included information such as age, sex, and site of sample recruitment. This study was conducted in accordance with the Declaration of Helsinki and the protocol was approved by the Ethics Committee of the Oswaldo Cruz Foundation (FIOCRUZ) under CAAE number 34049514.7.0000.5248.
Serological assaysSerum samples were evaluated using commercial immunoenzymatic assays to detect HBV serological markers HBsAg (MONOLISA HBs Ag ULTRA Assay, Bio-Rad, Marnes La Coquette, France), total anti-HBc (MONOLISA Anti-HBc PLUS Assay, Bio-Rad, Marnes La Coquette, France) and anti-HBs (MONOLISA Anti-HBs PLUS Assay, Bio-Rad, Marnes La Coquette, France) according to the manufacturer's instructions.
Nucleic acid extraction and detection of HBV DNAStandard laboratory procedures to avoid cross-contamination of samples were carried out, from the processing of the specimens to nucleic acid extraction and amplification, with the use of intercalated negative controls. Viral DNA from samples positive to serological markers HBsAg or anti-HBc alone was extracted from 200 µL serum using High Pure Viral Nucleic Acid Kit (Roche Life Science, Mannheim, Germany) according to the manufacturer's instructions. Detection of viral DNA was performed by a semi-nested PCR assay with a limit of detection of approximately 150 IU/mL using specific primers for partial S/Pol amplification (806 nt) and thermal cycling conditions as described by Mallory et al.15
Sequencing, genotyping, and phylogenetic relatednessThe generated PCR products were purified using the High Pure PCR Product Purification kit (Roche Life Science) and prepared for direct sequencing using BigDye Terminator 3.1 Cycle Sequencing Kit (Applied Biosystems, Foster City, CA, USA) along with internal sequencing primers as described by Mallory et al.15 Sequencing products were electrophoresed on an ABI 3730 DNA Analyzer (Applied Biosystems) and retrieved electropherograms from both strands were analyzed and edited using MEGA 7 software16 to generate consensus sequences.
HBV genotyping was performed by phylogenetic analysis of the 20 sequences determined in this study compared with a multiple sequence alignment of containing 22 HBV sequences representing all known genotypes available in the GenBank. Phylogenetic analysis was carried out using the maximum likelihood method (bootstrap re-sampling test with 1,000 replicates) in MEGA version 7 software16 under the model of nucleotide substitution GTR+G+I, which was selected as the best-fit model for the input multiple sequence alignment.
New datasets containing HBV subgenotype A1 sequences determined here and 22 highly similar sequences obtained through a search in database Nucleotide collection using Megablast algorithm available in BLAST (https://blast.ncbi.nlm.nih.gov/Blast.cgi) were analyzed in MEGA version 7 software to calculate genetic distances. In order to establish a more accurate phylogenetic relatedness between sequences from hemodialysis units, a Bayesian coalescent analysis was conducted using Markov Chain Monte Carlo (MCMC) statistical framework implemented in BEAST v1.10.4.17 The best-fitting substitution model for the dataset was GTR + G + I and several runs with different combinations of clock (strict, uncorrelated lognormal-relaxed, and uncorrelated exponential-relaxed clock) and demographic models (constant size, exponential growth, and Bayesian skyline) were performed. Each MCMC analysis was run for 5×107 generations to achieve the convergence of parameters, which was assessed after a 10% burn-in and a calculation of the effective sample size (ESS) using TRACER v1.7.1. Models were considered consistent when all parameter estimates showed ESS values > 200, and their uncertainty was reflected in the 95% Highest Posterior Density (HPD) intervals. To compare and define which model parameters are most likely to characterize our dataset, marginal likelihood estimation (MLE) using path sampling (PS)/stepping-stone sampling (SS) was performed to calculate Bayes factors of each analysis. The uncorrelated lognormal-relaxed clock and Bayesian skyline population models had the greatest statistical support and were selected as priors. After MCMC run and convergence of parameters, a maximum clade credibility (MCC) tree was summarized from the posterior distribution of trees with TreeAnnotator and plotted in FigTree v1.4.2 program.
Statistical analysisDemographic and epidemiological data (sex and age) were compiled along with serological and molecular tests results in a spreadsheet created in Microsoft Excel software, version 2019. Descriptive statistical analysis was performed with calculation of means and frequencies. Possible relationships between categorical variables were evaluated with the chi-square test for independence or Fisher's exact tests when appropriate. P-values < 0.05 were considered statistically significant. Statistical analyses were performed with GraphPad Prism software, version 9.0.
ResultsCharacteristics of the study populationThe present study was based on a convenience sampling that included three hemodialysis units from the metropolitan area of Rio de Janeiro. Based on the estimated prevalence of patients on dialysis in the state of Rio de Janeiro in 20172 (633 per million inhabitants, which would represent around 10,578 individuals), the present study, which included 644 individuals, would represent 6.1% of the total dialysis population in the state of Rio de Janeiro. Most of the individuals were male (346/644; 53.7%) and the mean age of the population was 50.3 ± 15.2 years, ranging from 14 to 89 years. The most frequent age group consisted of individuals aged between 40 to 59 years (277/644; 43%). From the 644 samples, 55.1% were collected in hemodialysis units in the city of Rio de Janeiro, whereas 26.7% and 18.2% were collected in the cities of Queimados and Niterói, respectively.
Prevalence of serological markers for HBV infectionThe presence of serological markers for HBV infection (HBsAg, anti-HBs, and anti-HBc) was evaluated in the studied population. A total of 219 individuals (34.0%) had no detectable HBV serological markers, meaning they were likely susceptible to infection. Current HBV infection (HBsAg+) was detected in 5.9% (38/644) of the dialysis patients. Vaccinated HBV individuals represented 26.6% of the study population (171/644), whereas resolved infection (anti-HBc+/anti-HBs+) was found in 30.7% (198/644). “Anti-HBc alone” status, indicating a previous contact with the virus with no detectable protective antibody (either due to low titers or ongoing occult HBV infection) was identified in 2.8% (18/644) of the participants. Vaccinated HBV status was statistically associated with age, sex and site of collection. Individuals aged less than 40 years, female and those recruited in Niterói city demonstrated a high frequency of HBV vaccination (Table 1).
Frequency of serological markers for hepatitis B in relation to age, sex, and location (city).
Samples from HBsAg-positive individuals (n = 38) were submitted to a qualitative PCR to investigate the presence of HBV DNA, which was successfully detected in 15 individuals (39.5%).
In order to identify cases of OBI among the patients, HBV DNA amplification was performed by qualitative PCR in 18 samples from “anti-HBc alone” individuals. A total of five patients had detectable HBV DNA, indicating a frequency of OBI of 27.8% in this group. All but one case of OBI was detected in male individuals with a mean age of 53.8 ± 14.8 years.
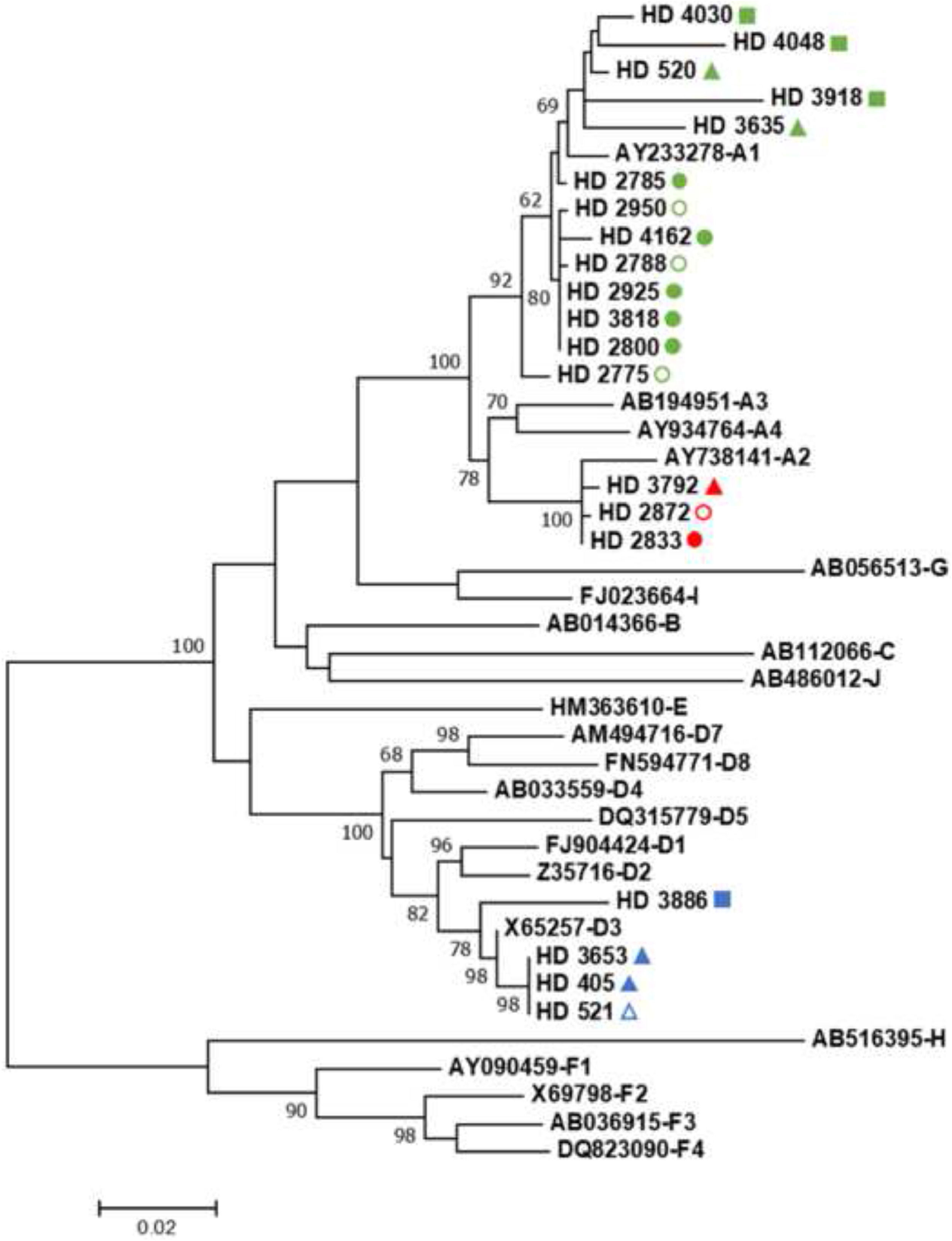
Phylogenetic analysis of these 20 sequences along with international HBV sequences representing all known genotypes classified most of the samples as genotype A (16/20; 80%), with the remaining four samples being classified as HBV/D (4/20; 20%). Considering HBV/A sequences, thirteen (13/16; 81.25%) belonged to subgenotype A1 and three sequences (3/16; 18.75%) to A2. All HBV/D sequences determined in the current study were classified as subgenotype D3. It is noteworthy that all three subgenotypes were also found in the sequences from OBI cases (three HBV/A1, one A2, and one D3), suggesting the possibility that this type of infection might be a potential source of viral dissemination in HUs (Fig. 1).
Maximum likelihood phylogenetic tree based on the GTR+G+I model for genotyping HBV strains found in CKD patients under hemodialysis. Sequences retrieved from CDK patients collected in the HU located in the city of Rio de Janeiro are represented with a circle whereas samples from HU units located in Niterói and Queimados are represented with a triangle and a square, respectively. Shapes in green, red, and blue, represent sequences from HBV/A1, A2, and D3, respectively. Filled and empty shapes represent sequences from HBsAg-positive individuals and OBI cases, respectively. HBV reference sequences are represented by their GenBank access number followed by genotype classification. Bootstrap values greater than 60% for the 1,000 replicates are represented in the nodes of the tree.
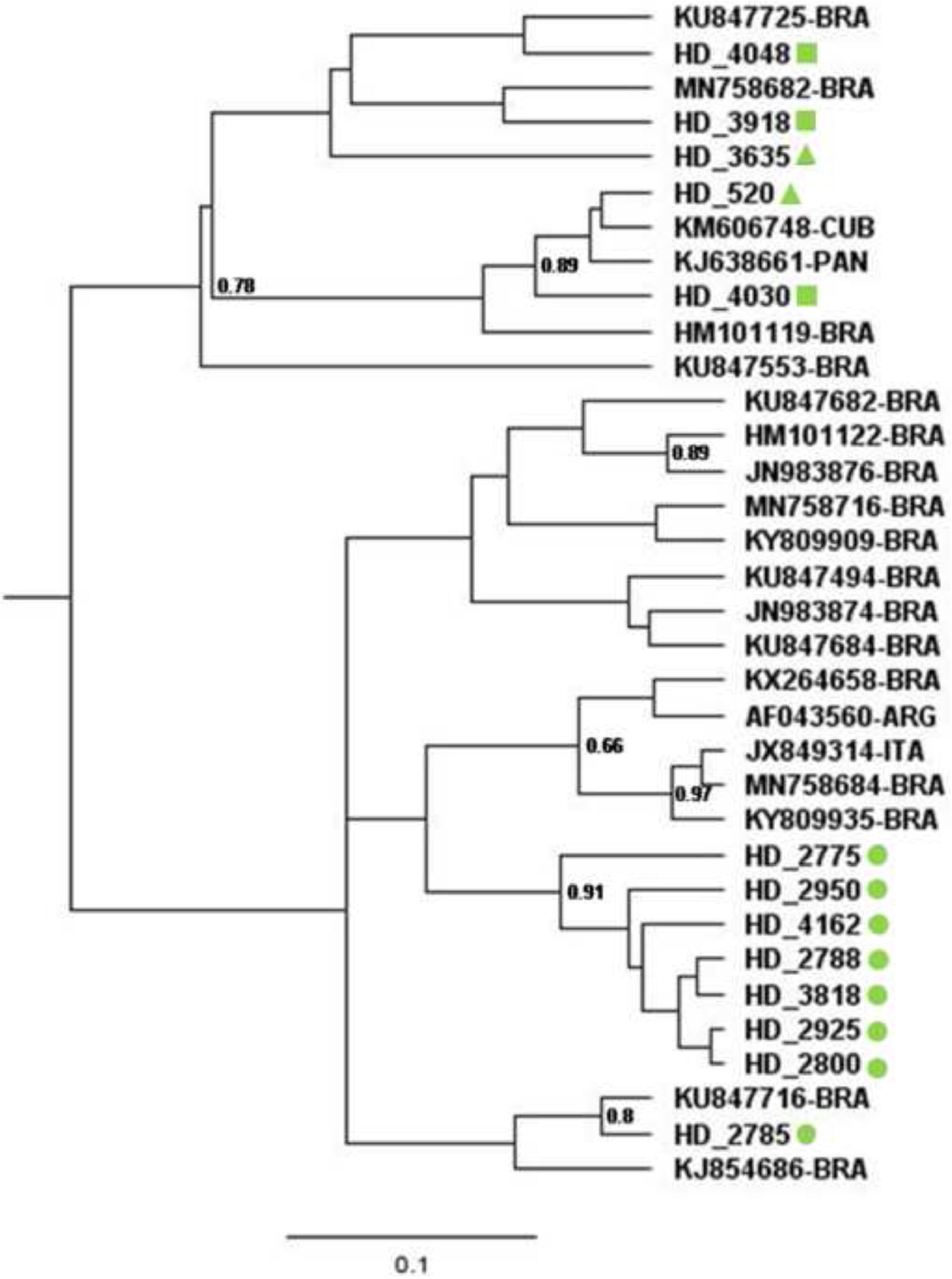
In order to obtain a more accurate analysis of the relatedness between HBV/A1 sequences found in CKD patients, a Bayesian Markov Chain Monte Carlo (MCMC) approach was applied in a set of 22 highly similar HBV/A1 sequences retrieved by BLAST. Bayesian Maximum Clade Credibility tree of HBV/A1 sequences (Fig. 2) indicated that seven of the eight sequences collected in the HU located in the city of Rio de Janeiro clustered together in a highly supported clade (posterior probability = 0.91). Furthermore, even considering an alignment containing the most similar sequences available in GenBank, genetic distance indicated a p-value two-fold lower within this clade (genetic distance = 0.0051 ± 0.0014) in comparison to the genetic distance within the remaining taxa (d = 0.0107 ± 0.0019), evidence that could suggest the occurrence of nosocomial spread of HBV in this HU. Regarding the other six HBV/A1 sequences from CKD patients, four of them (HD4048, HD3918, HD3635, and HD2785) were clustered with formerly reported Brazilian A1 sequences while two (HD520 and HD4030) were closely related to sequences from Cuba and Panama.
Bayesian Maximum Clade Credibility tree of HBV/A1 sequences. Sequences retrieved from CDK patients collected in the HU located in the city of Rio de Janeiro are represented with a green circle whereas samples from HU units located in Niterói and Queimados are represented with a green triangle and a green square, respectively. 22 highly similar HBV sequences retrieved from BLAST are represented by their GenBank access number followed by country of origin of the sequence in the two-letter country codes defined in ISO 3166-1.
Considering HBV/D3 sequences from CDK patients, one sample collected in the HU located in Queimados split apart from a branch that included three samples from the Niterói unit, which were identical along the sequenced fragment, pointing to the possibility of a contamination event in this unit (Fig. 1). Regarding HBV/A2 sequences and their relatedness, the high degree of similarity shared by this subgenotype in the given fragment prevented the establishment of a confident relationship between inquired taxa.
Analysis of deduced amino acid sequence coded by the amplified fragment indicated that none of the HBV S gene sequences of OBI cases had major mutations previously associated with OBI (sY100S/C, sS113N, sT126P/I/A, and sQ129R/L). HBV isolated from sample HD4048 had three amino acid substitutions in the S gene (sT118A, sK122R, and sF134/L) that are commonly reported in OBI strains.
DiscussionAccording to data from the 2018 census of the Brazilian Society of Nephrology, the prevalence of hepatitis B virus (HBV) in Brazilian hemodialysis units (HU) is 0.8%2. This rate should be analyzed with caution since only 38% of HU joined this national survey and previous Brazilian studies indicated infection rates that reached 15.4% in certain centers,14 even after HBV vaccination had become mandatory for all CKD patients undergoing hemodialysis in Brazil. The absence of official data on the proper implementation and coverage of such government ordinance, added to the lower vaccine seroconversion rate observed in CKD patients, reinforces the importance of continuous improvement of control safety measures in renal therapy to prevent the risk of infection and transmission of HBV, such as: (i) early diagnosis and periodic serological testing of hepatitis B markers, (ii) separate rooms for HBV carriers, (iii) proper waste disposal and cleaning and disinfection of hemodialysis machines, and (iv) full-vaccination schedules in patients under dialysis and health care workers.18
In the present study, we found a prevalence of 5.9% of CKD patients with detectable HBsAg, indicative of active viral infection. This prevalence in HUs from Rio de Janeiro is in accordance with the range of HBV infection reported in previous Brazilian studies. Former studies conducted in HUs located in other southeastern states – where most of CKD patients under dialysis are concentrated – reported a similar prevalence in the state of Minas Gerais (4.4%) as well as higher rates of infection in clinics in São Paulo (15.4%).14,19,20 Functional cure for HBV infection was found in 30.7% of CKD patients, similar to CKD patients from Tocantins, Santa Catarina, and Goiás States, which were 35.6%, 23.2%, and 29.7%, respectively.21,13,22 In general, the presence of anti-HBc in CKD patients reported by these studies is much higher than in the general population (5.35% - 12%),23-25 likely due to the high-risk of exposure to blood-borne infectious diseases during hemodialysis. Regarding immunity against HBV, it is noteworthy that more than 40% of the studied population was susceptible to HBV infection, which can have a major impact on viral transmission, morbidity, and mortality in this population with high risk of exposure to blood-borne diseases. Previous studies carried out in HUs in São Paulo, Santa Catarina, and Pernambuco reported that vaccination coverage was merely 35.3%, 15.4%, and 67.2% in CKD patients, respectively.13,26,27 These data demonstrate that mandatory vaccination of CKD patients implemented in 1996 has not been adequately implemented and requires urgent surveillance and awareness by HUs and users of the importance of HBV vaccination to prevent viral dissemination in this vulnerable group. Furthermore, even with the vaccination schedule of four doses of 40 micrograms each, seroconversion rate in CKD patients is lower than in the general population,28 highlighting the importance of periodically monitoring anti-HBs status in this group.
Assessing HBV serological profiles and age, sex and location, we found significant differences for vaccinated HBV individuals. Greater prevalence of vaccination was found in females, likely due to the more careful and preventive social behavior attributed to women compared to men. In addition, most vaccinated individuals were aged less 40 years old, reflecting the effectiveness of universal immunization in children and adolescents in the last 20 years.
HBV DNA was detected in 39.5% (15/38) HBsAg-positive CDK patients. Since the viral DNA amplification protocol relied on a sensitive PCR assay with a limit of detection of approximately 150 IU/mL, the absence of detectable HBV DNA in an overt hepatitis B infection might be related to the low and stable viral load over time in the dialysis population reported by previous studies,29,30 and/or a suppression in viral replication due to an ongoing antiviral treatment against HBV (information not available in our records). Most important in the context of a dialysis unit, absence of HBV DNA detection in a chronic patient indicates a very low risk of viral transmission.31
The prevalence of occult HBV infection in hemodialysis patients has been reported worldwide, showing great variation even in studies from the same country. While some studies reported high OBI prevalence in Spain (58%), Italy (26.6%), Greece (20.4%), and Egypt (26.9%), others indicated very low rates of infection (0%, 0%, 0.9%, and 3.8%, respectively).32
In this study, the prevalence of OBI in CKD patients with anti-HBc as a unique serological marker was 27.8%. A wide range of prevalence of OBI had been reported worldwide in this group, from 0.4% in Canada33 to 50% in Iran.34 These dissimilar rates might be explained by (i) the molecular approach employed and its sensitivity, (ii) the study population selection criteria, and (iii) distinct prevalence of HBV infection in specific geographic locations. Our findings indicated a much higher rate than reported by previous Brazilian studies regarding “anti-HBc alone” dialysis patients where the frequency of OBI was 0.5%, and 3%.26,35 In these cases, discrepancy in OBI rate might be related to differences in geographical locality and study population criteria.
Analysis of amino acid substitutions in the S gene indicated that none of the HBV S gene sequences had major mutations (sY100S/C, sS113N, sT126P/I/A, and sQ129R/L) previously reported to be associated with OBI cases.36-38 Only one sample (HD4048; subgenotype A1) had three mutations in the S gene (sT118A, sK122R, and sF134/L) commonly found in OBI strains reported elsewhere,38 indicating that factors other than mutations in the HBV envelope gene are implicated in the occult character of the infection.
Although the restricted geographic distribution of HBV genotypes has been changing over the years mainly due to intense migratory flows that favor the circulation of different genotypes in the same region,39 genotype characterization in certain populations can be epidemiologically relevant in tracking patterns of HBV transmission and the introduction of imported strains.8 Here, our findings indicated a predominance of HBV/A, specifically subgenotype A1, which was found in 13/20 (65%) of the sequences. All HBV/D sequences (20% of the total) were classified as subgenotype D3, a genotype distribution that reflects what was previously described for the general population in Rio de Janeiro.4,9,40 Phylogenetic and molecular analysis of HBV/A1, determined here together with highly similar sequences and the identical HBV/D3 samples, suggests the likely occurrence of nosocomial transmission within HUs, reinforcing the need for greater attention to prevention and safety measures. Moreover, the presence of OBI sequences in the likely “transmission cluster” warns of the possibility of undetected HBV infection by routine serological assays being the source of contamination in HUs. The absence of epidemiological data of the dialysis routine of the participants however, prevented further support for the occurrence of nosocomial transmission and constituted a limitation of this study, together with the impossibility of defining the HBV viral load and e antigen status that could provide useful information.
Occult Hepatitis B may have a major impact in transmission of infection by blood transfusion or organ transplantation.7 The occurrence of OBI cases in HUs affected by nosocomial transmission events sheds light on the importance of surveillance for blood-borne occult infections due to their potential risk of contamination in individuals constantly exposed to invasive procedures.
In conclusion, our data indicated a high prevalence of HBV infection in patients with CKD when compared to the general population. A significant proportion of individuals in our cohort was susceptible to HBV, reinforcing the need for expansion of vaccination coverage in this group in view of the inherent risk of infection in hemodialysis treatment. Moreover, the presence of closely related strains in the same HD unit highlighted the importance of continuous improvement of control safety measures in renal therapy and laboratory surveillance of serological markers to prevent the risk of infection and transmission of HBV.
Authors's contributionsStudy design, Livia Melo Villar; Data curation, Livia Melo Villar; Data analysis, Livia Melo Villar; Data collection, Ketlyn Araujo Fraga; Methodology, Ketlyn Araujo Fraga, Ana Carolina da Fonseca Mendonça, Juliana Custódio Miguel, Elisangela Ferreira da Silva, Bianca Cristina Leires Marques, Jakeline Ribeiro Barbosa, Paulo Sérgio Fonseca de Sousa, and Lia Laura Lewis-Ximenez; Project administration, Livia Melo Villar; Writing – original draft, Francisco Campello do Amaral Mello. All authors read and approved the manuscript.
FundingThis research was supported by Oswaldo Cruz Institute - Oswaldo Cruz Foundation/FIOCRUZ - Brazil.
The authors thank the DNA Sequencing Platform FIOCRUZ for performing nucleotide sequencing and Oswaldo Cruz Institute - Oswaldo Cruz Foundation/FIOCRUZ – Brazil for financial support.