Vancomycin-resistant enterococci colonization has been reported to increase the risk of developing infections, including bloodstream infections.
AimIn this study, we aimed to share our experience with the vancomycin-resistant enterococci bloodstream infections following gastrointestinal vancomycin-resistant enterococci colonization in pediatric population during a period of 18 months.
MethodA retrospective cohort of children admitted to a 400-bed tertiary teaching hospital in Izmir, Turkey whose vancomycin-resistant enterococci colonization was newly detected during routine surveillances for gastrointestinal vancomycin-resistant enterococci colonization during the period of January 2009 and December 2012 were included in this study. All vancomycin-resistant enterococci isolates found within 18 months after initial detection were evaluated for evidence of infection.
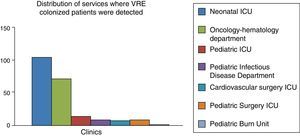
FindingsTwo hundred and sixteen patients with vancomycin-resistant enterococci were included in the study. Vancomycin-resistant enterococci colonization was detected in 136 patients (62.3%) while they were hospitalized at intensive care units; while the remaining majority (33.0%) were hospitalized at hematology-oncology department. Vancomycin-resistant enterococci bacteremia was present only in three (1.55%) patients. All these patients were immunosuppressed due to human immunodeficiency virus (one patient) and intensive chemotherapy (two patients).
ConclusionIn conclusion, our study found that 1.55% of vancomycin-resistant enterococci-colonized children had developed vancomycin-resistant enterococci bloodstream infection among the pediatric intensive care unit and hematology/oncology patients; according to our findings, we suggest that immunosupression is the key point for developing vancomycin-resistant enterococci bloodstream infections.
The emergence and spread of vancomycin-resistant enterococci (VRE) as a nosocomial pathogen represent a major health problem since its first isolation in the United Kingdom and in France.1,2 Recent articles reported a double digit number of hospitalizations for VRE infections between 2003 and 2006.3
Colonization is the key point for VRE infections. Infections generally follow VRE colonization mostly in gastrointestinal tract.4 VRE colonization was reported to increase a patient's risk of developing infections, such as bloodstream infections (BSIs).5,6 Another study reported a 5- to 10-fold increased risk of infection once a patient was colonized with VRE.7 The VRE infection rates were highest in hematologic–oncologic patients, organ transplant recipients and patients in intensive care units while it is reported to be nearly zero in immunocompetent patients. However, studies including children with VRE infections following gastrointestinal VRE colonization were rare and most data were adopted from adult studies.5,6,8–11
In this study, we report our experience with the VRE bloodstream infections following gastrointestinal VRE colorizations for an 18-month period.
Materials and methodsThis retrospective study was conducted in Dr. Behçet Uz Children's Hospital, a 400-bed tertiary teaching hospital in Izmir, Turkey, between January 2009 and December 2012. Patients with newly detected VRE colonization during routine surveillances for gastrointestinal VRE colonization were included in this study.
In our center; rectal sample screening for VRE was performed at admission and weekly in all intensive care units (ICUs); hematology–oncology department and neonatal intensive care unit using conventional cultures and molecular diagnostic techniques. The “red flag” precautions for patients with VRE colonization were also performed and strict infection control policies as part of patient management were applied in our center.
Using the VRE colonized patients cohort, we observed all the patients during 18 months after initial detection using in-patient and outpatient medical records at the same institution. The patients’ age, gender, service where patient was hospitalized, re-hospitalization, episodes of bacteremia, and isolated microorganism were recorded. Medical records were reviewed to identify the body site that had been colonized or infected when the initial VRE-positive culture sample was obtained and whether the detection represented colonization or infection on the basis of the Centers for Disease Control and Prevention criteria.12
All VRE isolates found within 18 months after initial detection were evaluated for evidence of discrete infection. Two trained reviewers separately verified whether infections represented distinct and unrelated events. Subsequent infections were described according to the infection site and days since initial detection. We determined the proportion of patients who subsequently developed VRE infection within the study cohort. The investigators obtained further information about the patients’ complaints by calling the patients’ family.
VRE detection: rectal swabs were directly inoculated onto a chromogenic agar plate (ChromID VRE agar bioMérieux, France) containing 8mg of vancomycin ml−1 and incubated at 36°C aerobically for 72h. Identification and antibiotic susceptibility tests were performed using the automated VITEK-2 system (bioMérieux, France) via Gram positive identification card, AST-P592, a supplementary E-test (bioMérieux, Durham, NC) and disk diffusion test according to the manufacturer's instructions. Van A and Van B resistance phenotypes were reported by the system on the basis of MIC values.
Statistical analysis was performed by using the Statistical Package for the Social Science (SPSS) software. Distribution of numeric variables was tested by both graphical methods and Shapiro–Wilk test. The difference between means of numeric variables was tested by Student's t test or Mann–Whitney U test, where appropriate. The difference between proportions was tested by Chi-Square or Fisher's exact test. p<0.05 was considered statistically significant.
ResultsTwo hundred and sixteen patients with VRE colonization were included in the study. The median age of the patients was two months ranging from 14 days to 16 years. One hundred and forty patients were male (64.2%).
Colonization was detected in 136 patients (62.3%) while they were hospitalized at ICUs, and 71 patients (33.0%) were hospitalized at the hematology–oncology department.
During the study period of 18 months, 17 VRE colonized patients identified in the first year of hospitalization and six patients who had been hospitalized in other centers for fever were not included in the analysis.
During the study period a total of 103 bacteria were isolated. Seventeen coagulase-negative staphylococci isolates were deemed to be contamination as they had been isolated only in one bottle of the culture sets. Among the 86 isolated microorganims, the most isolated bacteria was coagulase-negative staphylococci (32.5%), followed by Candida parapsilosis (12.7%), Escherichia coli (11.6%) and Klebsiella species (9.3%) (Fig. 1). Out of the 193 colonized VRE patients, VRE bacteremia was present only in three (1.55%) patients.
The three patients who had VRE bacteremia episodes were a 4-month old girl who had perinatal human immunodeficiency virus (HIV) infection, a 6-year old boy who had been under intensive chemotherapy for acute myeloblastic leukemia, and a 7-year old girl under acute lymphoblastic leukemia.
DiscussionEnterococci were reported to be the third most common organism causing BSI in hospitalized children.13,14 However, in our clinical experience, we have witnessed few VRE associated BSI. This retrospective study was designed to review our experience with VRE associated BSI following VRE-colonized patients and to identify risk factors associated with VRE BSI infection.
In our study, most patients with VRE colonization were neonates and pediatric hematology–oncology patients followed by patients in intensive care units. VRE colonization in Neonatal ICU (NICU) was reported more occasionally compared to the two wards above.15–19 In addition to the NICU, VRE colonization has been well-defined in pediatric hematology-oncology wards supporting our findings.20–22
In our study; the rate of VRE BSI among colonized patients was 1.55%, which was lower than the majority of reports suggesting VRE BSI rates ranging from 0% to 45% among colonized individuals, depending on the population studied.9,10,23–32 Brennen et al.23 reported that no VRE BSI was recorded in 36 colonized residents of a long-term care facility, supporting the variability of VRE BSI. In a study, including 52 patients with VRE colonization, two patients were reported to have VRE BSI.30 A recent study from Taiwan reported only two patients with VRE BSI out of 47 patients who acquired VRE during their ICU stays.31
Adult patients are more likely to have more serious comorbid conditions reported to increase VRE colonization and infections.32 However, a previous study including 768 patients reported that only 31 (4.0%) of the patients had VRE BSI.32 Moreover, 13.4% of the adult patients with malignancies and VRE colonization were reported to develop VRE BSI suggesting that malignancy is an important risk factor for VRE-BSI.9 Comparing to these adult studies, children had shown the same pattern of adults but with a low rate of VRE BSI development.
Two of our hematology–oncology patients and one HIV infected child had significant degree of immunosupression. Previous studies reporting higher rates of VRE BSI among colonized solid organ transplant patients (6.3–11.5%),27–29 colonized patients with cancer (13.4–29.3%),9,24,25 colonized bone marrow transplant recipients (26.7–34.2%),31,32 and colonized patients (45%)33 supported our findings. Immunosuppressed patients were reported to be a special risk group for severe VRE infections.11,34 Matar et al.25 reported that, among 99 VRE-colonized patients with cancer, 29 (29.3%) developed VRE BSI, and the majority of these (71%) were neutropenic at the time they developed their infection. Zaas et al.9 reported that, among colonized cancer patients, diabetes mellitus, undergoing a gastrointestinal procedure, acute renal failure, and exposure to vancomycin were significant risk factors for developing VRE BSI.
Since hematology–oncology patients were found to have VRE associated BSI more intense precautions should be taken in these wards, as recommended by the Society for Healthcare Epidemiology of America35 and by the Centers for Disease Control and Prevention in hematology–oncology guidelines.36
This study is limited by its retrospective design and unavailability of subtyping VRE by pulsed-field gel electrophoresis. Since data regarding the risk of VRE BSI among children are limited our findings will be helpful in discriminating risk groups for developing VRE BSI.
In conclusion, our study found that 1.55% of VRE-colonized children had developed VRE BSI. According to our findings, Pediatric ICU and hematology/oncology patients are under higher risk for VRE colonization, and immunosuppression is the key point for developing VRE bloodstream infections.
Conflicts of interestThe authors declare no conflicts of interest.