We assessed late onset sepsis (LOS) rates of neonates in a neonatal intensive care unit (NICU) before and after implementing an evidence-based bundle to prevent these infections in a country with poor resources.
MethodsWe evaluate trends of LOS between October 2010 and August 2012 in a large tertiary hospital in Brazil. We designed a protocol based of CDC guidelines for insertion of maintenance of central venous catheter targeted to reduction of bloodstream infections. During this period two major events occurred: a great increase of LOS rates in January months and relocation of the unit to a provisory place. Additionally we evaluated the risk factors and etiology of these infections.
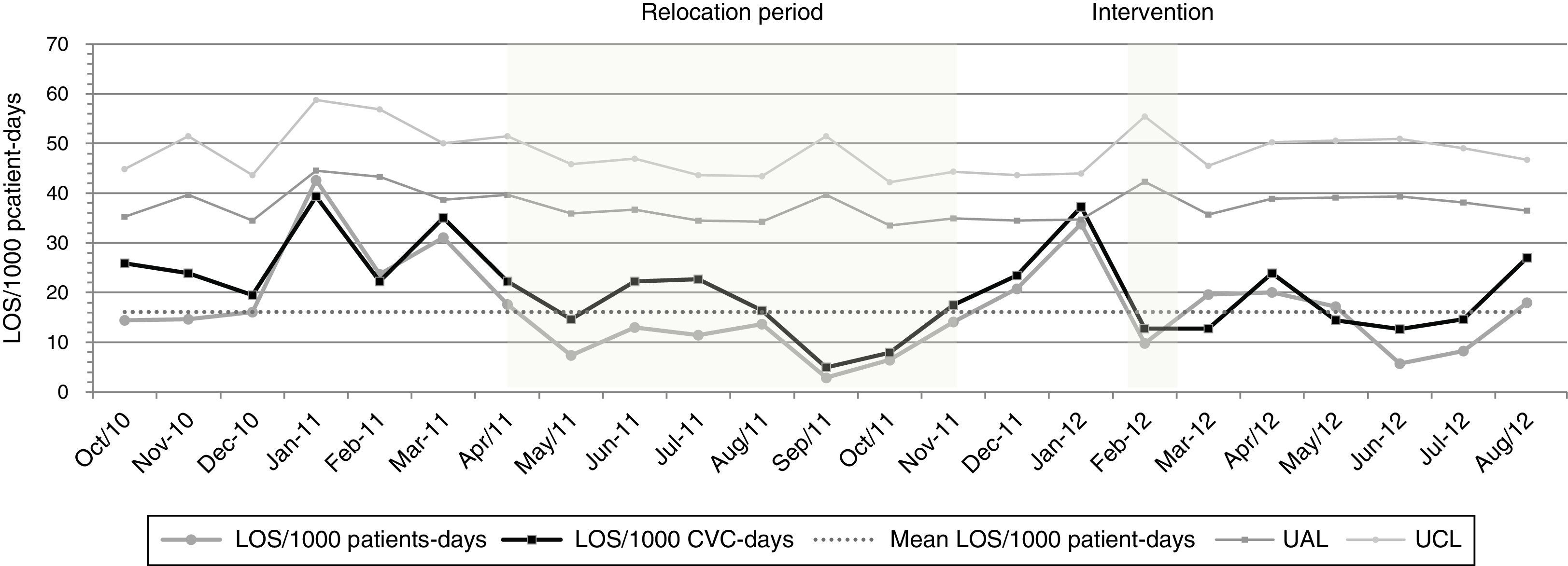
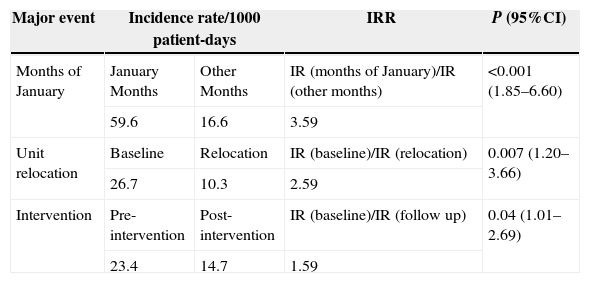
ResultsA total of 112 (20.3%) cases defined as LOS were found. The overall incidence rate of LOS in the study was 16.1/1000 patient/days and 23.0/1000 CVC-days. Our monthly rates data of LOS/1000 patient-day reveal fluctuations over the studied period, with incidence rates of these infections in staff vacation period (January 2011 and 2012) significantly higher (59.6/1000 patients-days) than compared with the other months rates (16.6/1000 patients-days) (IRR=3.59; p<0.001). As opposite, the incidence rates of LOS during relocation period was lower (10.3/1000 patients-days) when compared with baseline period 26.7/1000 patients-days (IRR=2.59; p=0.007). After the intervention period, these rates decreased in the post intervention period, when compared with preintervention 14.7/1000 patients-days and 23.4/1000 patients-days, respectively (IRR=1.59; p=0.04).
ConclusionThrough simple infection control measures, LOS can be successfully controlled especially in NICUs of limited resources countries such as ours.
Late-onset neonatal sepsis (LOS) remains an important cause of death, morbidity and long-term complications among premature infants, which are associated with prolonged hospital stay and increased health-care costs.1 It is of great interest to know the incidence and the strategies that are effective for preventing LOS in neonatal intensive care units (NICUs).2–4
The most common infections seen in neonates are central line-associated bloodstream infections (CA-BSIs), which substantially contribute to the burden and cost of neonatal care.5 One proposed approach to achieve low CA-BSI rates is implementation of catheter care bundles.6
Besides the classic risk factors for LOS, neonates requiring intensive care in developing countries are at high risk due to structural factors such as overcrowding, shortages of nursing and medical staff, lack or improper use of basic supplies and equipment, excessive use of antibiotics, insufficient knowledge and difficulties in the implementation of infection control practices.7–9
The aim of this study was to evaluate the changes in LOS incidence rates in a reference NICU at a Brazilian hospital considering different time periods: staff vacation period, relocation of the unit to a temporary site, and the effect of implementation of an evidence based care bundle.
Materials and methodsPatients and settingThe tertiary NICU of Hospital das Clinicas of Uberlandia city, Minas Gerais receives both inborn patients from high-risk pregnancies and patients referred from a wide surrounding region. The permanent NICU has 15 beds, rated level III (10 beds) and level II (5 beds) and admits an average of 500 infants each year. We designed a prospective interventional cohort study in the NICU including all infants admitted from October 2010 to August 2012.
Data collectionBlood cultures positive for any microorganism and/or with clinical symptoms of sepsis were actively and prospectively identified according to the National Healthcare Safety Network (NHSN) surveillance.10 Patients were followed from their admission to the unit their discharge or death. Standard definitions for health care associated infections were used.10 Laboratory information was required to identify all positive blood culture results. Medical records were reviewed to determine whether positive blood culture results met the criteria for LOS outlined by the National Healthcare Safety Network of the Centers for Disease Control (CDC),10 with assistance of a neonatologist from the unit. For all study patients the following characteristics were abstracted from clinical records: place of birth; gestational age; birth weight; length of total hospital stay; antibiotics use; invasive procedures such as central venous catheter (CVC), mechanical ventilation, and parenteral nutrition; and the following clinical scores: the Score of Neonatal Acute Physiology version II (SNAP II) and APGAR at five minutes of life. Data from patients with LOS were compared with those with no infection to compute risk factors associated with LOS.
InterventionThe intervention was implemented in February 2012. Practices primarily addressed interventions relating to the evaluation and prevention of central line-associated bloodstream infections. Literature was assessed for methodological quality and applicability, and based primarily on categories IA and IB by CDC guidelines, and the staff for CVC insertion and maintenance drafted a new protocol. These included complying with diagnostic criteria and standard techniques for monitoring nosocomial infections; improving hand-hygiene compliance; preventing intravascular catheter-related infections by adopting stringent insertion practices and catheter maintenance routines, including maintaining closed vascular systems and reliable, antiseptic methods for line insertion and maintenance (dressing changes, administration of medication, daily chlorhexidine application to the umbilical cord stump, daily catheter hub scrub with 70% isopropyl alcohol), dressing changes only when soiled, instead of routine weekly changes, using full-barrier precautions during insertion of central venous catheters, cleaning the skin with chlorhexidine 0.2%, avoiding the femoral site if possible, and removing unnecessary catheters besides better knowledge about prevention of CA-BSI in neonates. The intervention focused on group sessions and feedback on pathogenesis and data of CA-BSI per 1000 CVC days in the unit before intervention. All staff was invited to attend the meetings in order to review the infection rates and device use and care. A catheter checklist was created to ensure adherence to CA-BSI prevention practices at the time of CVC insertion. CVC insertion was observed by a nurse to ensure that aseptic technique was maintained. Otherwise, visual displays with A3-size color posters that emphasized care with CVC were distributed at strategic points of the unit.
Comparison ratesWe accessed the incidence of LOS in the NICU in different periods during the study: (1) January 2011 and 2012, staff vacation period; (2) April–October 2011, relocation of the unit to a temporary nursery for repairing the air conditioner system; and (3) February 2012, the intervention period designed to reduce bloodstream infection (intervention bundle). Rates during staff vacation were compared to rates of other months during the study (October 2010–December 2010; February 2011–March 2011; November 2011–December 2011), excluding relocation and post-intervention periods. Rates of LOS during the relocation period (from April 2011 to October 2011) were compared rates observed in the period before unit relocation (from October 2010 to March 2011, excluding January 2011). To evaluate the impact of the intervention bundle, the incidence rates of LOS during pre-intervention period (October 2010–December 2011, excluding January 2011 and relocation period) were compared to rates during the post-intervention period (February 2012–August 2012). A graph based on a Poisson probabilistic distribution was constructed for the monitoring of LOS per thousand patient-days, according to Arantes et al. (2003).11
Bacterial identificationCultures were processed using the BACT/Alert® (bioMérieux–Durham, USA). Microbial identification and antimicrobial susceptibility testing were performed on the VITEK II automated system (bioMérieux).
Statistical analysisThe incidence rate, expressed as the number of LOS episodes per 1000 patient-days for each month, was calculated including all admitted neonates to the unit. Total patient-days for a particular month was the sum of the total number of days all infants who stayed in the NICU in that particular month. Total CVC days was the sum of the total number of days with a CVC of all infants who stayed in the NICU in that particular month. The incidence of LOS was calculated for each month using patient-days and CVC-days in a particular month as the denominator and number of infections diagnosed during that month as the numerator. A 95% CI for each incidence rate was estimated using a Poisson distribution. The incidence rate ratio (IRR) for each event relative to the IRR of the follow-up and its 95% CI was calculated. Univariate analysis of risk factors for BSI was performed by applying the Chi-square test. Statistical significance level was set at p≤0.05. The variables with p≤0.05 in univariate analyses were included in the multivariate logistic regression model to identify independent risk factors for BSI. Software BioEstat (Version 5.0, Brazil) was used for multiple logistic regression, and GraphPad Prism (Version 5.0, San Diego, USA) was used for other analyses.
Ethical approval: Our study was evaluated and approved by the research Ethics Committee of the Federal University of Uberlandia under the number 464/2010.
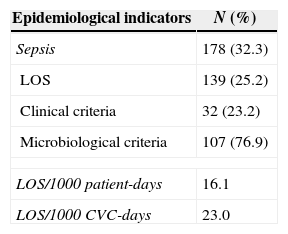
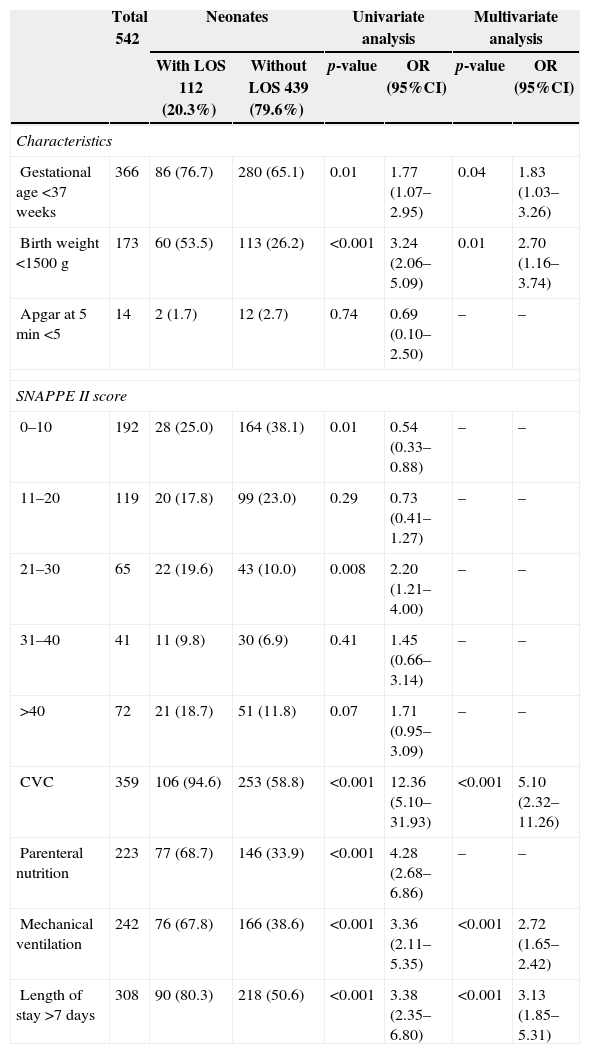
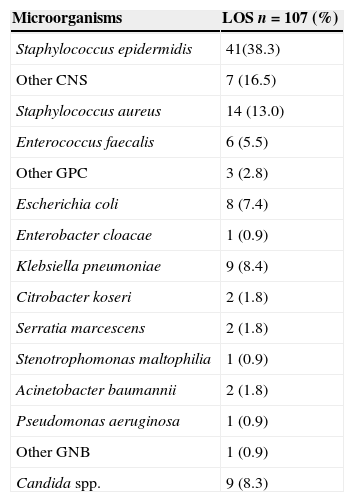
ResultsOut of 551 neonates admitted in the NICU during the study period, 112 neonates developed sepsis (20.3%) with a total of 178 episodes (32.3%); 139 (25.2%) was defined as LOS and only 38 (6.8%) as early onset sepsis (EOS). For LOS, 76.9% (107/139) of episodes had late positive blood culture (Table 1). The overall incidence rate of LOS in the study was 16.1/1000 patient-days and 23.0/1000 CVC-days. The mean length of stay was 18.8 days and CVC utilization density rate was 0.7. Univariate and multivariate analyses of potential risk factors for LOS are displayed in Table 2. Overall, gestational age<37 weeks (p=0.01) birth weight<1500g (p=0.001), use of CVC (p<0.001), parenteral nutrition (p<0.001), and mechanical ventilation (p<0.001) were risk factors for LOS in univariate analysis. The following risk factors remained independently associated with LOS: use of CVC (p<0.001), parenteral nutrition (p=0.05), and mechanical ventilation (p<0.001) (Table 2). Our data have shown that 66.3% (71/107) of LOS were caused by Gram-positive cocci, 25.1% (27/107) by Gram-negative bacilli, and 8.4% (9/107) by Candida sp. Staphylococcus epidermidis was the most frequent agent (37.9%), followed by Staphylococcus aureus (12.9%). Among Gram-negative bacilli, Klebsiella pneumoniae was the most frequent (8.3%). The pathogens isolated from LOS are shown in Table 3.
Epidemiological indicators for LOS in a NICU from October 2010 to August 2012.
| Epidemiological indicators | N (%) |
|---|---|
| Sepsis | 178 (32.3) |
| LOS | 139 (25.2) |
| Clinical criteria | 32 (23.2) |
| Microbiological criteria | 107 (76.9) |
| LOS/1000 patient-days | 16.1 |
| LOS/1000 CVC-days | 23.0 |
LOS, late-onset sepsis; CVC, central venous catheter; NICU, neonatal intensive care unit.
Risk factors for late-onset sepsis (LOS) in the neonatal intensive care unit from October 2010 to August 2012.
| Total 542 | Neonates | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|---|
| With LOS 112 (20.3%) | Without LOS 439 (79.6%) | p-value | OR (95%CI) | p-value | OR (95%CI) | ||
| Characteristics | |||||||
| Gestational age <37 weeks | 366 | 86 (76.7) | 280 (65.1) | 0.01 | 1.77 (1.07–2.95) | 0.04 | 1.83 (1.03–3.26) |
| Birth weight <1500g | 173 | 60 (53.5) | 113 (26.2) | <0.001 | 3.24 (2.06–5.09) | 0.01 | 2.70 (1.16–3.74) |
| Apgar at 5min <5 | 14 | 2 (1.7) | 12 (2.7) | 0.74 | 0.69 (0.10–2.50) | – | – |
| SNAPPE II score | |||||||
| 0–10 | 192 | 28 (25.0) | 164 (38.1) | 0.01 | 0.54 (0.33–0.88) | – | – |
| 11–20 | 119 | 20 (17.8) | 99 (23.0) | 0.29 | 0.73 (0.41–1.27) | – | – |
| 21–30 | 65 | 22 (19.6) | 43 (10.0) | 0.008 | 2.20 (1.21–4.00) | – | – |
| 31–40 | 41 | 11 (9.8) | 30 (6.9) | 0.41 | 1.45 (0.66–3.14) | – | – |
| >40 | 72 | 21 (18.7) | 51 (11.8) | 0.07 | 1.71 (0.95–3.09) | – | – |
| CVC | 359 | 106 (94.6) | 253 (58.8) | <0.001 | 12.36 (5.10–31.93) | <0.001 | 5.10 (2.32–11.26) |
| Parenteral nutrition | 223 | 77 (68.7) | 146 (33.9) | <0.001 | 4.28 (2.68–6.86) | – | – |
| Mechanical ventilation | 242 | 76 (67.8) | 166 (38.6) | <0.001 | 3.36 (2.11–5.35) | <0.001 | 2.72 (1.65–2.42) |
| Length of stay >7 days | 308 | 90 (80.3) | 218 (50.6) | <0.001 | 3.38 (2.35–6.80) | <0.001 | 3.13 (1.85–5.31) |
CVC, central venous catheter; LOS, late-onset sepsis; OR, odds ratio.
Pathogens isolated from blood cultures in cases of late-onset sepsis (LOS) from October 2010 to August 2012.
| Microorganisms | LOS n=107 (%) |
|---|---|
| Staphylococcus epidermidis | 41(38.3) |
| Other CNS | 7 (16.5) |
| Staphylococcus aureus | 14 (13.0) |
| Enterococcus faecalis | 6 (5.5) |
| Other GPC | 3 (2.8) |
| Escherichia coli | 8 (7.4) |
| Enterobacter cloacae | 1 (0.9) |
| Klebsiella pneumoniae | 9 (8.4) |
| Citrobacter koseri | 2 (1.8) |
| Serratia marcescens | 2 (1.8) |
| Stenotrophomonas maltophilia | 1 (0.9) |
| Acinetobacter baumannii | 2 (1.8) |
| Pseudomonas aeruginosa | 1 (0.9) |
| Other GNB | 1 (0.9) |
| Candida spp. | 9 (8.3) |
LOS, late-onset sepsis; CNS, coagulase negative Staphylococcus; GPC, Gram-positive cocci; GNB, Gram-negative bacilli.
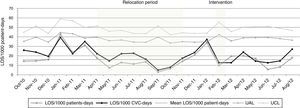
Our monthly rates of LOS/1000 patient-days reveal fluctuations over the study period (Fig. 1), with incidence rates of these infections during staff vacation periods (January 2011 and 2012) significantly higher (59.6/1000 patient-days) when compared with rates during other months (16.6/1000 patient-days) [IRR=3.59; p<0.001]. In contrast, the incidence rates of LOS during the relocation period were lower (10.3/1000 patient-days) when compared with rates of the baseline period (26.7/1000 patient-days) [IRR=2.59; p=0.007]. Table 4 shows that post-intervention rates (14.7/1000 patients-days) decreased in relation to pre-intervention rates (23.4/1000 patient-days) [IRR=1.59; p=0.04]. Nonetheless, there was no significant difference in gestational age, SNAPPE-II score, low birth weight, and APGAR<5 at five minutes, in all these periods.
Comparison of incidence rates of LOS during the months of January, time of unit relocation, and quality improvement bundle.
| Major event | Incidence rate/1000 patient-days | IRR | P (95%CI) | |
|---|---|---|---|---|
| Months of January | January Months | Other Months | IR (months of January)/IR (other months) | <0.001 (1.85–6.60) |
| 59.6 | 16.6 | 3.59 | ||
| Unit relocation | Baseline | Relocation | IR (baseline)/IR (relocation) | 0.007 (1.20–3.66) |
| 26.7 | 10.3 | 2.59 | ||
| Intervention | Pre-intervention | Post-intervention | IR (baseline)/IR (follow up) | 0.04 (1.01–2.69) |
| 23.4 | 14.7 | 1.59 | ||
IR, incidence rate; IRR, incidence rate ratio.
The present study demonstrates that through infection control measures, LOS can be successfully controlled temporally, even in a NICU with high endemic levels of S. epidermidis. Although the overall incidence of LOS in our NICU was higher than rates reported in developed countries12 they are similar to rates reported in developing countries including other settings in Brazil.13,14
Our approach placed great emphasis and value on local context, considering that the technical intervention on CVC insertion reduces infection rates, its introduction and application reflect the different conditions among staff of each NICU.
The main aim of this study was to evaluate the effectiveness of a care bundle designed to reduce LOS in the unit. Over the surveillance period, we identified two events that significantly increased and reduced, respectively, the rates of LOS: staff vacations (months of January) and the unit relocation to a temporary nursery. According to literature, there is an association between nurse staffing and outcome of healthcare-associated infections in infants. Ensuring that staff receives infection control training is important to guarantee that the NICU is adequately staffed.15,16 In the months of January there was less trained nurse staff in the unit, due to their vacation period, with a subsequent increase of bloodstream infections rates. We hypothesize that inadequate nurse staffing and increased workload in an intensive care unit, can result in low hand-hygiene compliance, breaks in aseptic technique, or compromises in practice that might increase the risk of infection. Other studies from western countries have demonstrated a direct link between staffing levels and infection rates in NICUs, mainly due shortage of trained healthcare workers.16,17
It should also be pointed out the decrease of infection rates observed in this study when the unit was transferred to an improvised area, probably because of more isolation facilities, more space per cot, and enforcement of hand washing compliance during in that temporary place. These aspects have also been shown in other studies with impact on infection rates.18,19
Despite some unexpected events during the study period, we could demonstrate an important reduction in LOS rate among the neonates after a sustained effort in February 2012 that were maintained at lower rates over five months, probably due the implementation of the bundle. Our results are consistent with previous reports showing reductions in overall NI multifaceted interventions, and especially those related to central lines.20,21 We have shown that specific education and a protocol adopting the recommended procedures for CVC insertion and care besides feedback to healthcare workers (HCWs) can result in significant reduction of CA-BSI rates. In our NICU, earlier approach by our team, using simple and inexpensive intervention to increase compliance with evidence-based infection control practices, decrease CA-BSI rates.4 After implementation of these measures, the overall CA-BSI rate declined from 32% to 19.6%, and from 37.3% to 15.2% in low birth weight neonates.
All these strategies described so far were based on our findings from the surveillance study that detected high incidence of LOS in the unit associated to S. epidermidis, a major causative agent of these infections in our NICU. From our data it was possible to design a case–control study to detect the main risk factors associates to LOS in critical neonates. Thus, in this report the multivariate analysis identified low birth weight, length of stay in the unit >7 days, use of CVC, mechanical ventilation, and parenteral nutrition as independent risk factors for BSI, similar to those found in other studies.22,23 The central venous catheters and low birth weight placed infants at a higher risk of developing LOS, as has been shown in other studies.22
The organisms causing neonatal infections often differ from one place to another and may change over time within the same setting. In this study most of LOS was microbiologically confirmed, and S. epidermidis was identified as a major cause of LOS in the unit. Gram-positive bacteria, particularly coagulase negative Staphylococci, are the most common cause of LOS in many industrialized as well as in some developing countries.24 Gram-negative bacillary and Candida infections have become another major cause of neonatal sepsis, as seen in our study when around one-fourth of the episodes were caused by BGN, mainly K. pneumoniae, with a high case fatality and long-term complications.14,25
Our study has several limitations. First, we had two main events, summer outbreaks and unit relocation that interfered in the analysis of the baseline period of intervention. Second, we were not able to ensure the adoption of the checklist to achieve stable compliance in order to evaluate the real adherence and ascertain the full impact of the bundle. Third, our study was performed at a single unit, although representative of Brazilian teaching hospitals, but the results may not be applicable to other units. Finally, both the intervention and post-intervention periods has lasted only one and six months, respectively, and efforts of short duration may not be as successful as years of sustained strategies reduce LOS.
In conclusion, the incidence of LOS in our NICU is high, in both overall as well as in low birth weight neonates, reflecting the situation in resource-poor countries and show that application of evidence-based practices can lead to significant reduction in LOS rate. Additionally, the analysis of trends over a period of 22 months showed an increase of LOS rates during staff vacation months and a reduction of LOS rates during the relocation of the unit. Finally, these infections are preventable, and their elimination presents an opportunity to improve patient outcomes and reduce costs in the unit. Lessons learned about the importance and feasibility of an individualized local approach on specific techniques to decrease CA-BSI as evidenced in our proposed bundle that describes simple and inexpensive practices that lead to the reduction of these infections in the NICU.
Conflicts of interestThe authors declare no conflicts of interest.
The authors would like to thank the financial support by Brazilian Funding Agency FAPEMIG (Fundação de Amparo à Pesquisa do Estado de Minas Gerais).