Human T-lymphotropic viruses (HTLV) are Deltaretroviruses that infect millions of individuals worldwide via the same transmission routes as HIV. With the aim of exposing the possible re-emergence of HTLV in West Africa since discovery, a systematic review was carried out, focusing on the distribution of the virus types and significance of frequent indeterminate reports, while highlighting the need for mandatory routine blood screening. Capturing relevant data from discovery till date, sources searched were Google Scholar, CrossRef, NCBI (PubMed), MEDLINE, Research Gate, Mendeley, abstracts of Conferences and Proceedings, organization websites and reference lists of selected papers. A total of 2626 references were initially retrieved using search terms: Worldwide prevalence of HTLV, HTLV in Africa, HTLV in West Africa, HTLV subtypes, HTLV 3 and 4 in Africa, HTLV of African origin, HTLV seroindeterminate results, Spread of HTLV. These references were rigorously trimmed down to 76. Although evidence shows that HTLV is still endemic in the region, West Africa lacks recent epidemiological prevalence data. Thorough investigations are needed to ascertain the true cause of indeterminate Western Blot results. It is imperative that routine screening for HTLVs be mandated in West African health care facilities.
Human T-lymphophotropic virus (HTLV), formerly called human T-cell lymphotropic virus or human T-cell leukemia/lymphoma virus, is a member of the Deltaretrovirus genus. The Deltaretroviruses belong to the Retroviridae family and include bovine leukemia virus (BLV) and simian T-lymphotropic virus (STLV), aside HTLVs.1
Retroviruses were not isolated from humans prior to 1979. Hence, HTLV (type 1) was the first human retrovirus to be isolated1 from a patient with cutaneous T-cell lymphoma. HTLV (type 2) was isolated a few years later from a patient with hairy cell leukemia.2 It was then believed that HTLV-2 could be associated with hairy cell leukemia. However, the failure to isolate HTLV-2 from replicable number of hairy cell leukemia patients proved that it was not the etiological agent of hairy cell leukemia but rather, a passenger agent.
The third and probably the most important retrovirus was discovered a year later and placed in the same genus as HTLV-1 and 2 viruses. Upon subsequent research however, it was renamed human immunodeficiency virus and reclassified under the Lentivirus genus. In 2005, researchers discovered two (2) new HTLV types – HTLV-3 and HTLV-4.3,4 Knowledge about these viruses is limited as few cases have been reported, compared to HTLV 1 and 2.
HTLV 1 is endemic in some parts of the world (Southwestern Japan, South America, the Caribbean Basin, the Middle East, Australo – Melanesia, the West Indies, Jamaica), as well as equatorial Africa,5 where West Africa lies. HTLV-2 is endemic in pockets of populations. With the aim of exposing the possible re-emergence of HTLV in West Africa, this review focuses on the distribution of the virus types, points out the significance of frequent indeterminate reports, while highlighting the need for mandatory routine blood screening prior to blood donation and/or transfusion.
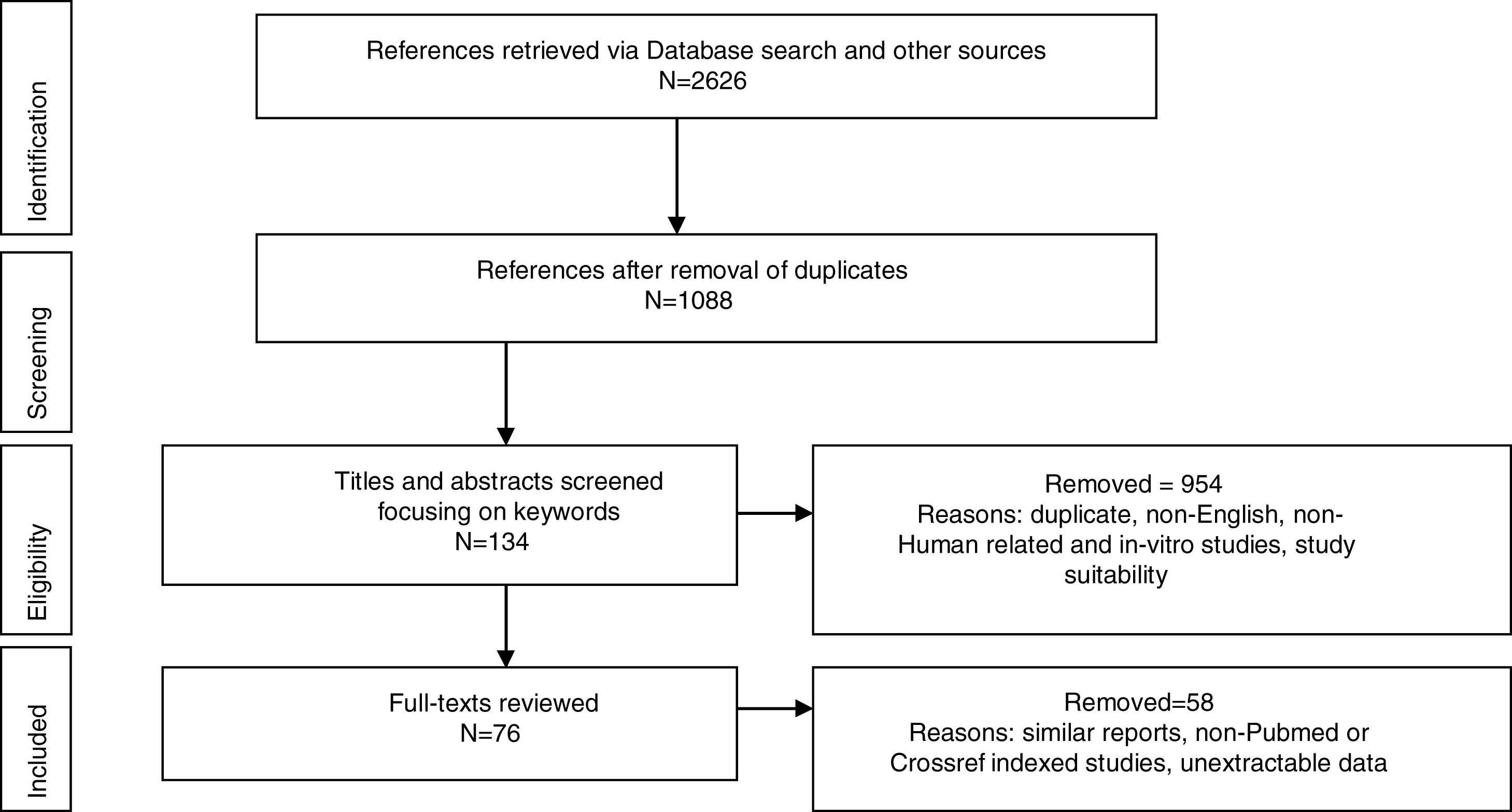
MethodologyPreferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) was used to report the identified studies. Google Scholar, CrossRef, NCBI (PubMed), MEDLINE, Research Gate, Mendeley were searched from database inception until February 2018. Abstracts of Conferences and Proceedings, organization websites and reference lists of selected papers were also searched. Search terms were: Worldwide prevalence of HTLV, HTLV in Africa, HTLV in West Africa, HTLV subtypes, HTLV 3 and 4 in Africa, HTLV of African origin, HTLV seroindeterminate results, spread of HTLV. The first 100 sources identified by Crossref and Google Scholar were screened. Duplicate titles were removed (Fig. 1). Eligible studies included original reports from prevalence studies, case studies and cohort studies. Titles and abstracts recovered in the search were screened for study suitability, focusing on the keywords; non-English, non-Human related, and in-vitro studies were excluded at this point and full-text copies of papers that possibly dealt with the review topic were retrieved. Same reports of already reported population by another study were also excluded. The retrieved data were screened and extracted by NCJ and checked independently by A, EE and M for any discordance. Study characteristics are presented in figures and table.
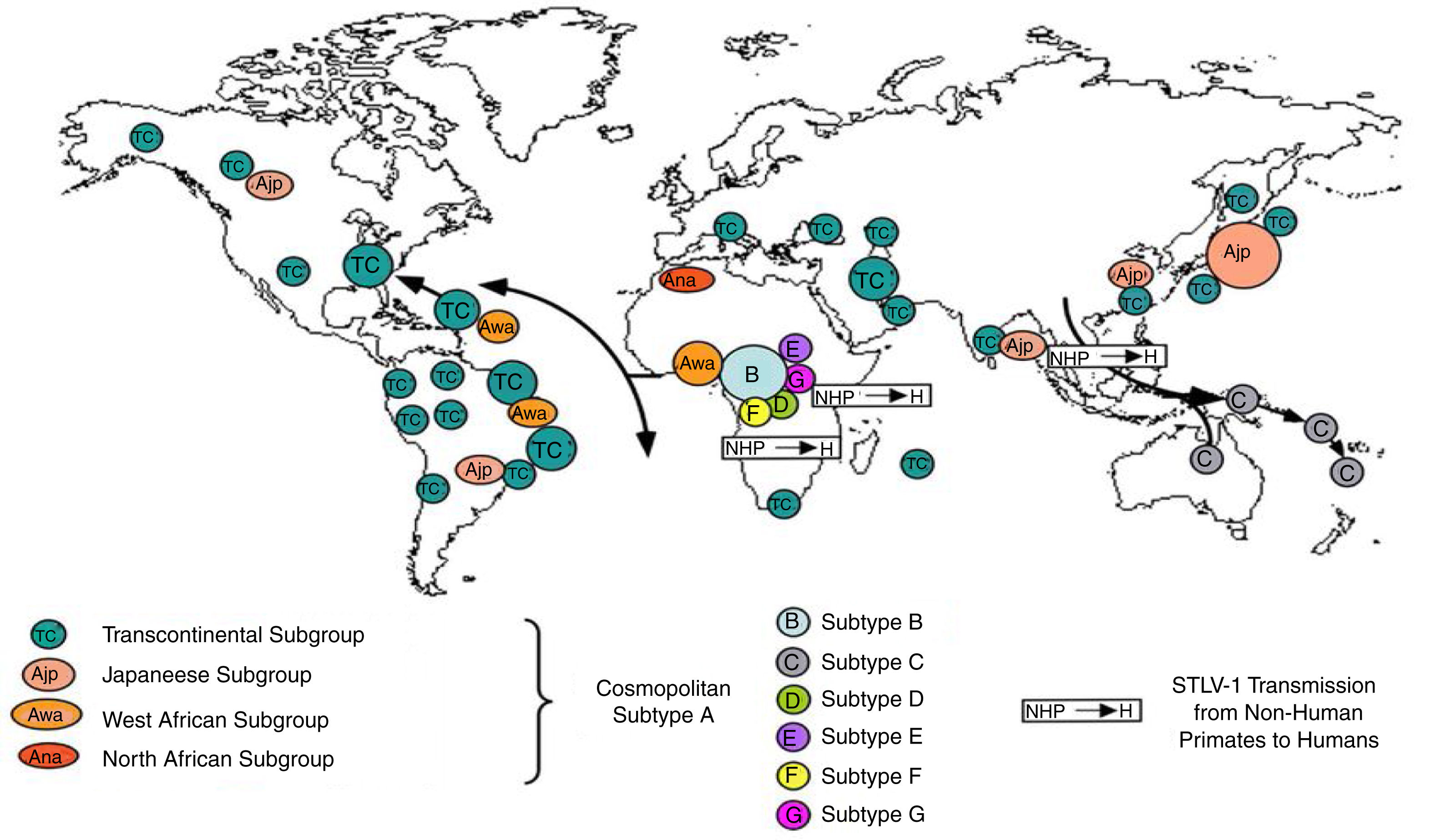
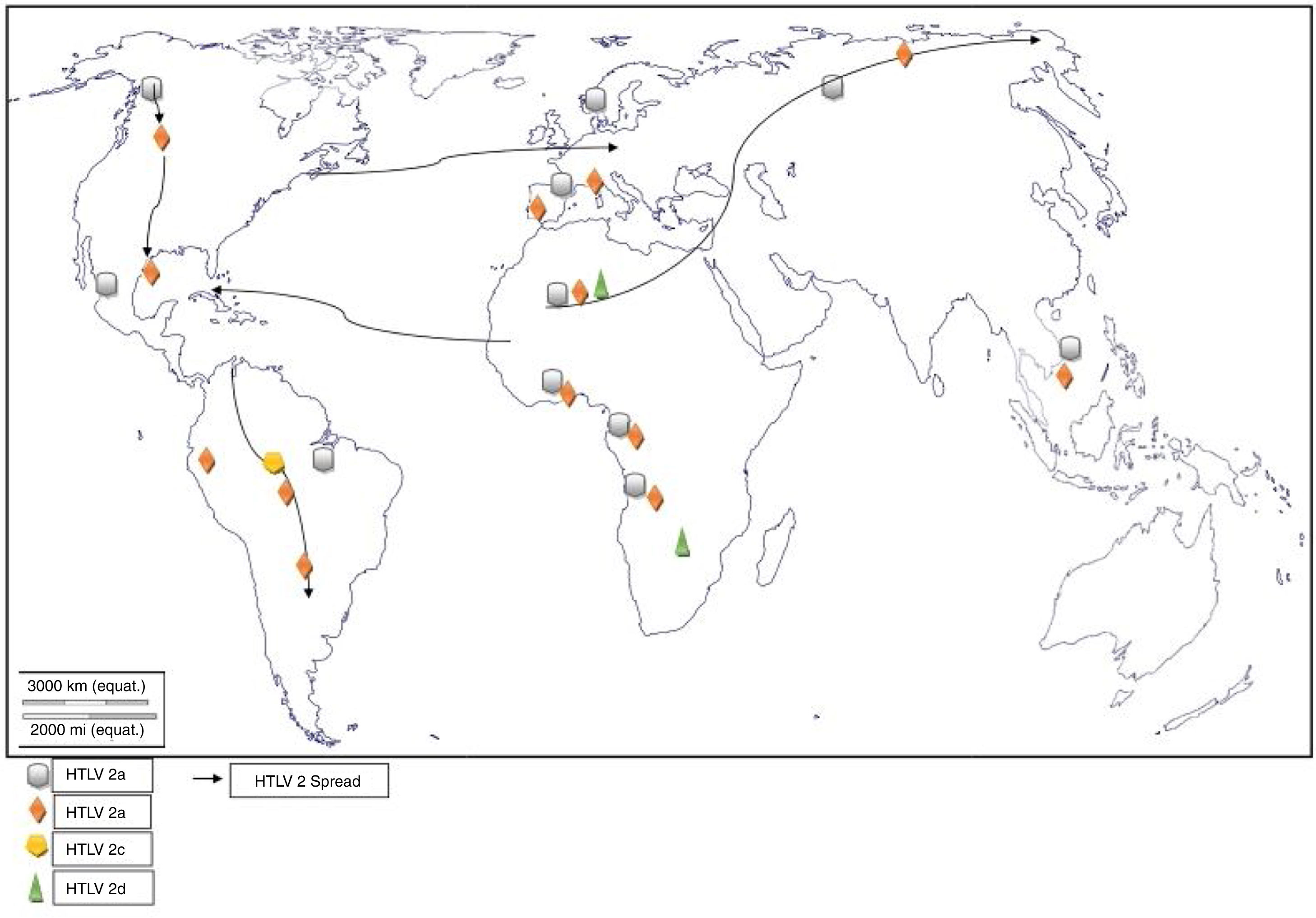
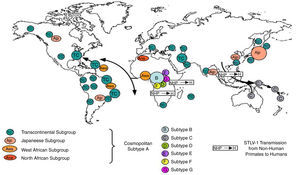
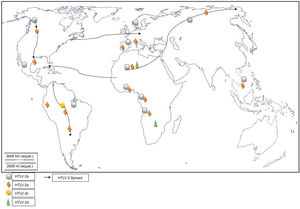
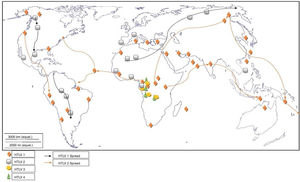
Worldwide distribution of HTLVsThe HTLV-1 and 2 viruses have experienced gradual but considerably consistent increase in prevalence since their discovery. HTLV-1 subtypes are associated with specific regions of the globe (Fig. 2), while HTLV-2 subtypes are related to highly specific subpopulations (e.g. Brazilian Indians) and behaviors such as injection drug use (Fig. 3).
There are seven geographical or ethnic-related subtypes of HTLV-1 including the Cosmopolitan subtype A with four (4) subgroups, the Central African subtype B, the Australo – Melanesian subtype C, the Central African/Pygmies subtype D, the Central African subtypes E (also in Southern Africa), F, and G respectively.5 HTLV-2 has four known subtypes – A, B, C, and D.
Levine et al.6 carried out a study on the worldwide distribution of HTLV-1 virus on 43,445 participants (excluding endemic regions of Japan) and reported a prevalence rate of 3.7%. According to the study, the highest prevalence rate of 11.2% was seen in Hawaii, which was closely followed by West/Central Africa with a prevalence rate of 10.0%. The Caribbean basin had a prevalence rate of 5.1% while the other countries captured in the study had prevalence rates lower than 3.7% – South Africa (3.5%), Central/South America (2.9%), non-endemic regions of Japan (2.8%), Middle East (2.2%), Asia (excluding Japan – 1.4%), Continental North America (1.2%), with Europe having the least prevalence rate of 1.0%. The prevalence of HTLV-1 is different for different parts of the world. It is generally categorized into three strata – regions of low (less than 1%), average or moderate (1% to 5%), and high (greater than 5%) prevalence rates. Europe has been captured in many epidemiological studies, with most of the studies focusing on the United Kingdom, France and Spain.5 Most of the people diagnosed with HTLV were either born in – or had genetic affiliations with Afro – Caribbean and African origin, indicating that HTLV infections and/or transmission may be linked to human migration. In Asia, the prevalence of HTLV-1 has been reported to be between 0.098% and 2.12% in endemic areas of Iran and 0.3% and 37.0% in endemic areas of Japan.5,7,8 Irrespective of the fact that Japan is being bordered by PR China, North and South Korea, the Philippines, Northern Mariana Islands, and the Republic of China (Taiwan), these countries are largely free from HTLV infection, suggesting that the virus is not transmitted by migrants from these regions, thus ruling out human migration as the cause of endemicity in Japan.
The global distribution of human T-lymphotropic virus 2 (HTLV-2) is not as widespread as HTLV 1, as it is found to be present in pockets of populations. The virus is common among intravenous drug users (IDUs) in Eire, Spain, Italy and Scandinavia, less common in the UK, and rare in Germany and France.9,10 Subtypes A and B are endemic in IDUs and indigenous people of in North, South and Central (Latin) America, Europe and Asia (Vietnam, Italy and Spain),10 and occur sporadically in parts of West and Central Africa – Ghana, Gabon, Cameroon, where it was first isolated and Democratic Republic of Congo (DR Congo) among the pigmy tribes.2 Subtype C, which is a distinct molecular subtype, was isolated from the Amazon region of the Brazilian sub-cluster, while subtype D, distinct and genetically different from the other subtypes was isolated from Central African Congolese Efe Bambuti pygmy.10 It is possible that HTLV-2 was introduced into the IDUs population of the United States during the 1970s, and into Europe, slightly later.
Both HTLV-1 and 2 have been found to be involved in increasing epidemic in some factions of the world. However, HTLV-2 spread and/or prevalence is more common than HTLV-1 in the United States, although the overall prevalence is 200 per 100,000 population.11 Generally, the highest prevalence of HTLV-1 is found in Japan (37%) while Latin America is estimated to have the lowest prevalence (0.024–1.00%). Ample epidemiological studies in Turkey are lacking hence, the inability to categorize its stratum of prevalence. On the other hand, HTLV-2 (subtype C) is most endemic among the indigenous people of Brazil, hence, the Brazilian Amazon is the highest area of its endemicity in the world.9 An alarmingly high HTLV-2 prevalence of up to 61% has been found in Venezuela among the Yaruro and Guahibo populations9,10 while up to 3.8% prevalence has been found in Peruvian Amazon.
Seroprevalence of HTLV-1 is more predominant in females than males, indicating that females are more at risk of the retroviral infection.1 Vertical transmission however, accounts for higher male predisposition to HTLV-1 seropositivity at childhood. The ratio of adult T-cell leukemia/lymphoma (ATLL) is 2:1 in males and females while the reverse is the case for HTLV-1-associated myelopathy (HAM)/tropical spastic paraparesis (TSP).12 It is rather difficult to determine the gender distribution of HTLV-2 because of the peculiarity of the study populations, although it has been associated with the female gender in the United States.9 The prevalence of the viruses is higher in older age, majorly due to their long latency period. The time of expression of the viruses leading to malignant or neurological disorders, however, varies with individuals. ATLL is mostly rapidly progressive and fatal, with median survival time of two years. HAM/TSP may ensue as early as five months after transfusion-transmitted HTLV-1 infection.
There seems to be an overall decline in the worldwide distribution of HTLV-1 virus from 10–20 million to 5–10 million.5 The reason for such wide gap and difference in distribution may be due to the fact that large regions had not been investigated, few population-based studies are available, and the assays used for HTLV-1 serology were not specific enough at the time of early epidemiological studies.5 It should however, be noted that there is still a large amount of data yet to be captured in some areas of the world, as highly populated regions including East Africa, China, India, and the Maghreb, are hitherto yet to be exhaustively surveyed. Hence, the worldwide distribution of HTLV-1 may be slightly or well above 10 million infected individuals. Furthermore, the true prevalence of HTLV-1 worldwide has not been covered previously as epidemiological studies mostly cover only blood donors, pregnant women, or hospital based studies of different selected patients or high-risk groups such as IDUs, HIV, and other hematologic patients, neurologic patients or prostitutes, rather than the general population (villages, towns, cities, states, provinces regions or geo-political zones of a country).
Distribution in AfricaMolecular phylogenetic analyses have traced HTLV-1 to zoonotic origin with inter-species transmission from non-human primates to humans13 during the upper Paleolithic era; and transcontinental spread from Africa to Austro-Melanesia and Asian, down to North and South America (Fig. 2). The molecular characterization of HTLV-2 isolated from Cameroonian pygmy tribes also supported their African origin, similar to HTLV-1 (Fig. 3). HTLV infections have been identified in various regions, especially the sub-Saharan Africa. The most affected areas are East Africa, Central Africa and West Africa. Report on Africa shows HTLV-1 prevalence to be between 6.6% and 8.5% in Gabon, 1.05% in Guinea, 3.2% in Congo, 5.5% in Nigeria, 2.7% and 19.5% in Kenyan women.14 The prevalence lies between 0.5% and 4.2% in Ghana, 0.9% in Cameroon, 1.5% in Mozambique, 0.6% in Central African Republic, 1% in South Africa,5 0.63% in Malawi,15 and >15% in Seychelles.16
The initial isolations of HTLV-2 in African population were from pygmy tribes both from Ethiopia and West Africa.2,17 The identification of HTLV-2 – like primate virus in Central Africa suggests that the virus, like HTLV-1, originated from Africa. However, it is more widespread throughout the Americas than the African population, raising a few questions. A serosurvey across Africa2 established HTLV-2 prevalence rates of 14% from Bambuti pygmies in DR Congo and 2.3% from pygmies in Cameroon. HTLV-2 subtype B has also been characterized from Gabonese family,18 as well as Cameroonian pygmy of Bakola tribe. With an exception of a report of HTLV-2 infection in a new world primate in Mongolia, there is no population based evidence of the virus migrating from Old world to the New world. Other reports of HTLV-2 infection include: 0.1–0.5% in Gabon,19–22 6.47%23 and 0.1%24 in Ghana (Table 1), 0.08% in Guinea-Bissau,25,26 0.5–3.3% in Nigeria27–29 and 0.02% in Senegal.30
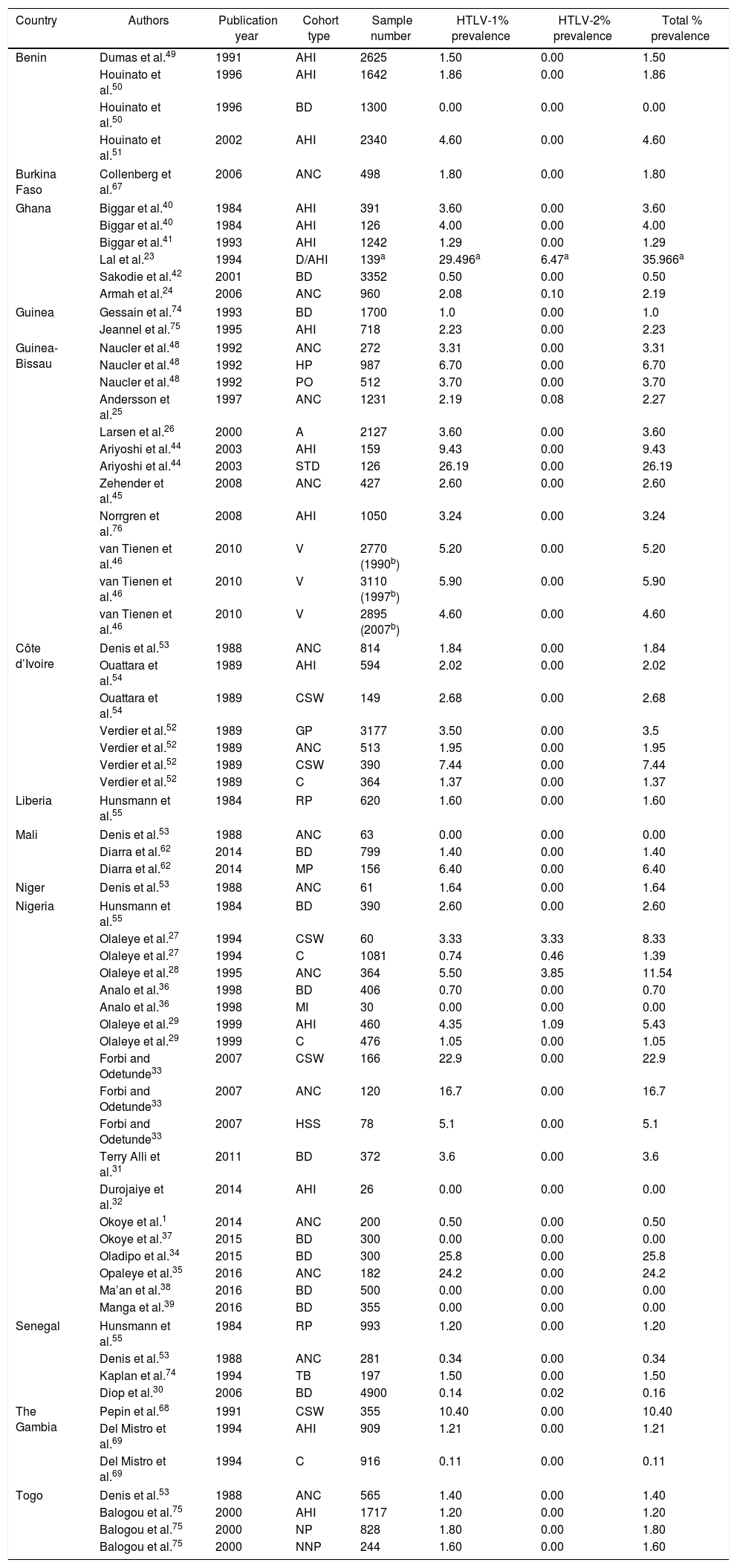
Reports of HTLV Prevalence in West Africa.
| Country | Authors | Publication year | Cohort type | Sample number | HTLV-1% prevalence | HTLV-2% prevalence | Total % prevalence |
|---|---|---|---|---|---|---|---|
| Benin | Dumas et al.49 | 1991 | AHI | 2625 | 1.50 | 0.00 | 1.50 |
| Houinato et al.50 | 1996 | AHI | 1642 | 1.86 | 0.00 | 1.86 | |
| Houinato et al.50 | 1996 | BD | 1300 | 0.00 | 0.00 | 0.00 | |
| Houinato et al.51 | 2002 | AHI | 2340 | 4.60 | 0.00 | 4.60 | |
| Burkina Faso | Collenberg et al.67 | 2006 | ANC | 498 | 1.80 | 0.00 | 1.80 |
| Ghana | Biggar et al.40 | 1984 | AHI | 391 | 3.60 | 0.00 | 3.60 |
| Biggar et al.40 | 1984 | AHI | 126 | 4.00 | 0.00 | 4.00 | |
| Biggar et al.41 | 1993 | AHI | 1242 | 1.29 | 0.00 | 1.29 | |
| Lal et al.23 | 1994 | D/AHI | 139a | 29.496a | 6.47a | 35.966a | |
| Sakodie et al.42 | 2001 | BD | 3352 | 0.50 | 0.00 | 0.50 | |
| Armah et al.24 | 2006 | ANC | 960 | 2.08 | 0.10 | 2.19 | |
| Guinea | Gessain et al.74 | 1993 | BD | 1700 | 1.0 | 0.00 | 1.0 |
| Jeannel et al.75 | 1995 | AHI | 718 | 2.23 | 0.00 | 2.23 | |
| Guinea-Bissau | Naucler et al.48 | 1992 | ANC | 272 | 3.31 | 0.00 | 3.31 |
| Naucler et al.48 | 1992 | HP | 987 | 6.70 | 0.00 | 6.70 | |
| Naucler et al.48 | 1992 | PO | 512 | 3.70 | 0.00 | 3.70 | |
| Andersson et al.25 | 1997 | ANC | 1231 | 2.19 | 0.08 | 2.27 | |
| Larsen et al.26 | 2000 | A | 2127 | 3.60 | 0.00 | 3.60 | |
| Ariyoshi et al.44 | 2003 | AHI | 159 | 9.43 | 0.00 | 9.43 | |
| Ariyoshi et al.44 | 2003 | STD | 126 | 26.19 | 0.00 | 26.19 | |
| Zehender et al.45 | 2008 | ANC | 427 | 2.60 | 0.00 | 2.60 | |
| Norrgren et al.76 | 2008 | AHI | 1050 | 3.24 | 0.00 | 3.24 | |
| van Tienen et al.46 | 2010 | V | 2770 (1990b) | 5.20 | 0.00 | 5.20 | |
| van Tienen et al.46 | 2010 | V | 3110 (1997b) | 5.90 | 0.00 | 5.90 | |
| van Tienen et al.46 | 2010 | V | 2895 (2007b) | 4.60 | 0.00 | 4.60 | |
| Côte d’Ivoire | Denis et al.53 | 1988 | ANC | 814 | 1.84 | 0.00 | 1.84 |
| Ouattara et al.54 | 1989 | AHI | 594 | 2.02 | 0.00 | 2.02 | |
| Ouattara et al.54 | 1989 | CSW | 149 | 2.68 | 0.00 | 2.68 | |
| Verdier et al.52 | 1989 | GP | 3177 | 3.50 | 0.00 | 3.5 | |
| Verdier et al.52 | 1989 | ANC | 513 | 1.95 | 0.00 | 1.95 | |
| Verdier et al.52 | 1989 | CSW | 390 | 7.44 | 0.00 | 7.44 | |
| Verdier et al.52 | 1989 | C | 364 | 1.37 | 0.00 | 1.37 | |
| Liberia | Hunsmann et al.55 | 1984 | RP | 620 | 1.60 | 0.00 | 1.60 |
| Mali | Denis et al.53 | 1988 | ANC | 63 | 0.00 | 0.00 | 0.00 |
| Diarra et al.62 | 2014 | BD | 799 | 1.40 | 0.00 | 1.40 | |
| Diarra et al.62 | 2014 | MP | 156 | 6.40 | 0.00 | 6.40 | |
| Niger | Denis et al.53 | 1988 | ANC | 61 | 1.64 | 0.00 | 1.64 |
| Nigeria | Hunsmann et al.55 | 1984 | BD | 390 | 2.60 | 0.00 | 2.60 |
| Olaleye et al.27 | 1994 | CSW | 60 | 3.33 | 3.33 | 8.33 | |
| Olaleye et al.27 | 1994 | C | 1081 | 0.74 | 0.46 | 1.39 | |
| Olaleye et al.28 | 1995 | ANC | 364 | 5.50 | 3.85 | 11.54 | |
| Analo et al.36 | 1998 | BD | 406 | 0.70 | 0.00 | 0.70 | |
| Analo et al.36 | 1998 | MI | 30 | 0.00 | 0.00 | 0.00 | |
| Olaleye et al.29 | 1999 | AHI | 460 | 4.35 | 1.09 | 5.43 | |
| Olaleye et al.29 | 1999 | C | 476 | 1.05 | 0.00 | 1.05 | |
| Forbi and Odetunde33 | 2007 | CSW | 166 | 22.9 | 0.00 | 22.9 | |
| Forbi and Odetunde33 | 2007 | ANC | 120 | 16.7 | 0.00 | 16.7 | |
| Forbi and Odetunde33 | 2007 | HSS | 78 | 5.1 | 0.00 | 5.1 | |
| Terry Alli et al.31 | 2011 | BD | 372 | 3.6 | 0.00 | 3.6 | |
| Durojaiye et al.32 | 2014 | AHI | 26 | 0.00 | 0.00 | 0.00 | |
| Okoye et al.1 | 2014 | ANC | 200 | 0.50 | 0.00 | 0.50 | |
| Okoye et al.37 | 2015 | BD | 300 | 0.00 | 0.00 | 0.00 | |
| Oladipo et al.34 | 2015 | BD | 300 | 25.8 | 0.00 | 25.8 | |
| Opaleye et al.35 | 2016 | ANC | 182 | 24.2 | 0.00 | 24.2 | |
| Ma’an et al.38 | 2016 | BD | 500 | 0.00 | 0.00 | 0.00 | |
| Manga et al.39 | 2016 | BD | 355 | 0.00 | 0.00 | 0.00 | |
| Senegal | Hunsmann et al.55 | 1984 | RP | 993 | 1.20 | 0.00 | 1.20 |
| Denis et al.53 | 1988 | ANC | 281 | 0.34 | 0.00 | 0.34 | |
| Kaplan et al.74 | 1994 | TB | 197 | 1.50 | 0.00 | 1.50 | |
| Diop et al.30 | 2006 | BD | 4900 | 0.14 | 0.02 | 0.16 | |
| The Gambia | Pepin et al.68 | 1991 | CSW | 355 | 10.40 | 0.00 | 10.40 |
| Del Mistro et al.69 | 1994 | AHI | 909 | 1.21 | 0.00 | 1.21 | |
| Del Mistro et al.69 | 1994 | C | 916 | 0.11 | 0.00 | 0.11 | |
| Togo | Denis et al.53 | 1988 | ANC | 565 | 1.40 | 0.00 | 1.40 |
| Balogou et al.75 | 2000 | AHI | 1717 | 1.20 | 0.00 | 1.20 | |
| Balogou et al.75 | 2000 | NP | 828 | 1.80 | 0.00 | 1.80 | |
| Balogou et al.75 | 2000 | NNP | 244 | 1.60 | 0.00 | 1.60 | |
Key: V, variable; C, children; A, adults; AHI, apparently healthy individuals; CSW, commercial sex workers; MI, malignant individuals; BD, blood donors; ANC, antenatal care (pregnant) women; HSS, high school students; PO, police officers; STD, sexually transmitted disease infected individuals; D, individuals infected with disease; MP, mistransfused patients; GP, general population; RP, rural population; HP, hospitalized patients; NP, neurological patients; NNP, non-neurological patients; TB, patients with tuberculosis.
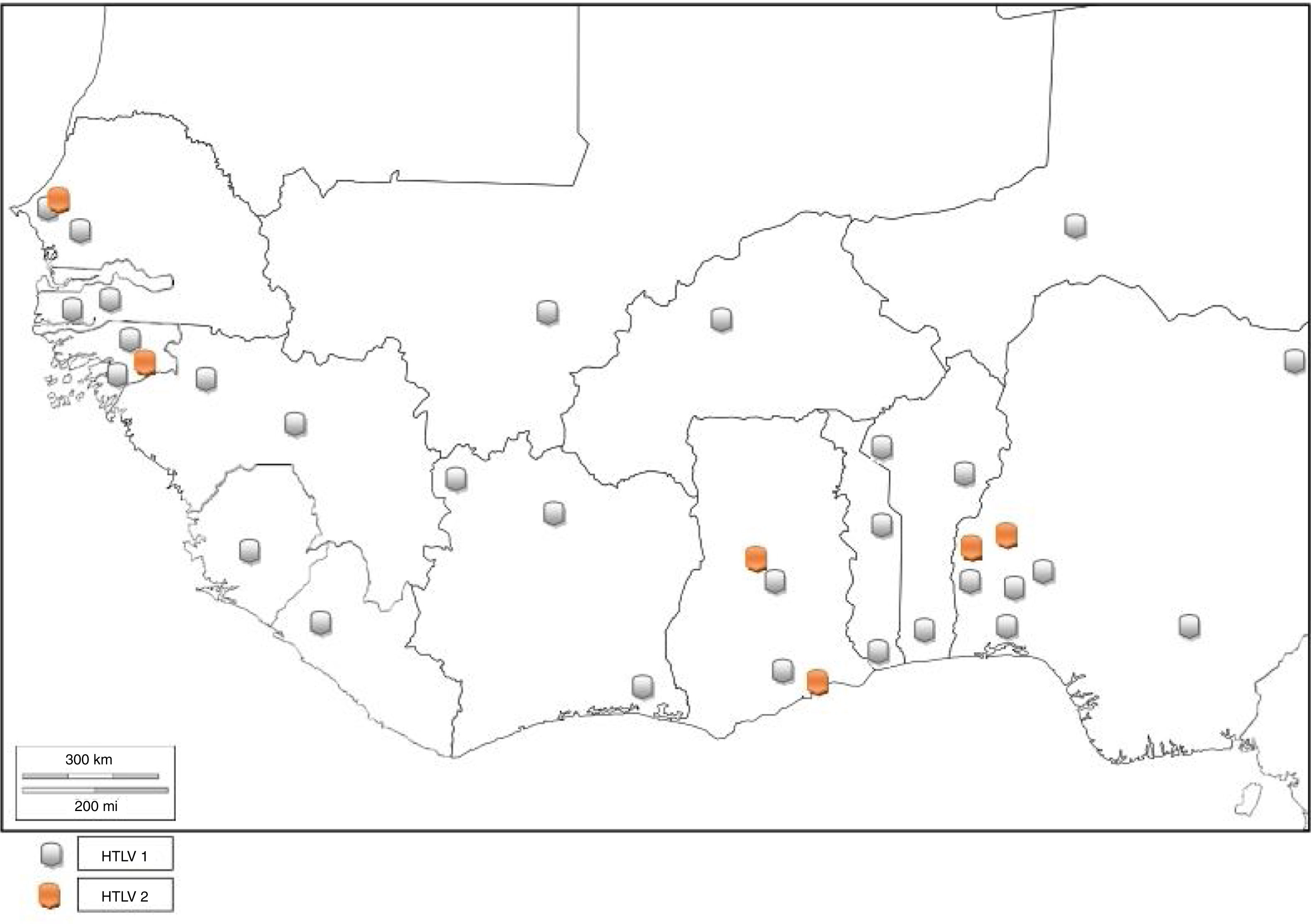
HTLV and associated diseases are not regarded as public health problem in West Africa, and are thus neglected. However, several studies have reported high prevalence (from 0% to 25%) of HTLV infection.
NigeriaIn 2011, Terry et al.31 found 3.6% seroprevalence of HTLV among blood donors in Oshogbo, South-Western, Nigeria. Durojaiye et al.32 found 0.5% prevalence among blood donors at a tertiary center in Lagos, Nigeria. All the enlisted donors had no history of blood transfusion; hence, no association was established between transfusion history and HTLV seropositivity. A cross-sectional study carried out in South-eastern Nigeria1 showed a prevalence rate of 0.5% in pregnant women. In South-Western Nigeria, an alarmingly high prevalence of 22.9% and 16.7% was observed among commercial sex workers (CSW) and pregnant women, respectively,33 while 5.1% prevalence was found in high school students. High prevalence rates of 25.8% and 24.2% were also identified among blood donors34 and pregnant women.35 Zero prevalence has also been reported in some Nigerian cohorts.32,36–39 This is an indicator that the prevalence rate of HTLV in Nigeria varies with location. Cases of dual infection with both HTLV 1 and 2 have also been reported in Nigeria.27,28 There is limited data on HTLV-2 in Nigeria as compared to HTLV-1.27–29 This could be because HTLV-1 has received more research attention in epidemiological and case studies, hence being the more studied virus type. It could also be that HTLV-2 was not found in the HTLV-1 and 2 pooled studies, indicating that the virus type may have little or no prevalence in the Nigerian population. There are no reported cases of HTLV-3 or HTLV-4 infection in Nigeria. The complete list of HTLV prevalence as reported by different authors is found in Table 1.
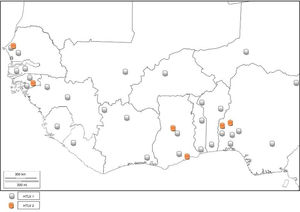
GhanaThe prevalence of HTLV-1 was assessed in two communities in the Ghanaian population a few years after the discovery of the virus.40 The prevalence rates were 3.6% and 4.0% in urban and rural populations, respectively. Prevalence increased with age (being 5.9% among persons above I0 years of age) but did not show any difference with sex. A population based study carried out between 1989 and 1990 detected the presence of HTLV-1 in Ghana.41 The specific prevalence rate was however, difficult to define due to high frequency of indeterminate results on western immunoblotting. The seropevalence was found to be between 1 and 2%, with no association between infectivity and malignancy or sexual behavior (prostitution). The reported seroprevalence of HTLV-1 among healthy Ghanaian blood donors has been stated to be between 0.4 and 4.2%.24,42 Armah et al.24 found HTLV-2 prevalence of 0.10% and 0.21% HTLV-1/2 among pregnant women. Lal et al.23 reported 29.49% HTLV-1 prevalence and 6.47% HTLV-2 prevalence from reactive individuals of previous serosurvey, while 3.59% positives were untyped (Table 1). HTLV co-infection with HIV has also been seen in Ghana43 (Fig. 5).
Guinea-BissauStudies have shown 3.60%, 26.19%, and 2.60% HTLV-1 prevalence among urban adult population, STD patients, and pregnant women of Guinea-Bissau, respectively.26,44,45 van Tienen et al.46 reported HTLV-1 prevalence to be 5.2% in 1990, 5.9% in 1997 and 4.6% in 2007, while noting a continued association with HIV (Table 1). In a survey carried out on the rural population, it was discovered that the Cosmopolitan HTLV-1 1aD subtype was predominant47 in the rural Bissau community. HTLV-1 co-infection with HIV-2 has been observed among hospitalized patients, police officers, and pregnant women in Guinea-Bissau48 as well as in rural populations to be ≤15%. There is higher mortality in HIV-2/HTLV-1 co-infected patients with tuberculosis compared to their HTLV seronegative counterparts, as the median CD4+ count is higher in the former. The endemicity of HTLV has declined to about 2%.
BeninBenin Republic was not unaffected by the early endemicity of HTLV-1/2, as a prevalence rate of 1.5% was reported between 1988 and 1989 in a population based study.49 Prevalence was lower in coastal region than in the north. Subsequent survey in the north observed 1.86% seroprevalence of HTLV-150 among apparently healthy individuals of the general population, while zero prevalence was seen among blood donors. A higher seroprevalence of 4.6% was reported in the same region in 1998 using Lot Quality Assurance Sampling (LQAS) method, which identified 25 (69.4%) communes with prevalence higher than 4.0%.51 A proband study identified a seroprevalence of 27.5% among 138 relatives of 32 infected subjects and 1.4% among 142 relatives of 32 control subjects. The annual incidence density was thus, reported to be 6%.50 There is limited data on the prevalence of HTLV in Benin Republic as few epidemiological studies have been carried out, and no recent study has been conducted. Other virus types have not been reported (Fig. 5).
Côte d’IvoireOnly early epidemiological data of HTLV prevalence are available; a cross-sectional serologic survey identified 3.5% prevalence of HTLV-1 in the general population. Neonates and children had 1.6% and 1.0% prevalence, respectively. The highest prevalence rates were observed in lepers (13.7%), female prostitutes (7.4%) and patients with neurologic syndromes (5.8%). The association between HTLV-1 and leprosy was however, not ascertained; 1.9% of the pregnant women were HTLV-1 seropositive.52 A previous survey on pregnant women observed 1.84% prevalence in urban and rural Ivorian women.53 HTLV-1 prevalence averaged 1.0–2.7% in the different regions,54 without significant increase in sexually overexposed groups. There is no report of HTLV-2, 3 and 4.
LiberiaAn early epidemiological study identified the prevalence of HTLV-1 in Liberia to be 1.6%.55 No further HTLV prevalence studies have been carried out in Liberia. However, a study carried out on Spanish immigrants identified HTLV-2 in a Liberian native.56 There was a recent report of HTLV-1 seropositivity in a patient with typical HAM/TSP who was born in Liberia but now resident in the United States.57 This is indicative that HTLV-1 and 2 are still existent in Liberia (Fig. 5).
Sierra LeoneIn a hospital sample in Sierra Leone, a patient was presumed to have HTLV-1 uveitis.58 Cosmopolitan strains of HTLV-1 from America, Caribbean, Japan, Polynesia and Equatorial DR Congo are said to have diverged from (Indo-Malay) Asian STLV-1 strains from Indian macaques (Macaca mulatta) to African baboons (P. hamadryas and Papio cynocephalus), before diverging from African STLV-1 strains of which strains from Sierra Leonean common chimpanzee, CH (Pan troglodytes) are among.59 Despite these, there are no available data on HTLV epidemiology in the Sierra Leonean population.
MaliHTLV-1 infection of Malian Origin has been documented60 and cases of adult T-cell leukemia/lymphoma (ATLL) have been characterized among Malian patients.61 An early study observed zero prevalence in a small cohort of pregnant women.53 Co-infections with HIV-2 and Strongyloides stercoralis are plausible. A survey carried out among blood donors and mistransfused patients yielded 1.4% (Table 1) and 6.4% HTLV-1 prevalence, respectively.62 HTLV-2, 3 and 4 have not been reported.
Niger RepublicIn an early study, 1.64% prevalence rate was seen among pregnant women from rural area.53 Develoux et al.63 identified a case of tropical spastic paraparesis associated with HTLV-1 in Niger Republic. No recent HTLV epidemiological survey has been carried out in the Nigerien population. However, a 55-year old male donor of Nigerien origin was found to be HTLV-1 positive in an Israeli study.64 Other HTLV types have not been reported (Fig. 5).
GuineaOnly two studies have reported the prevalence of HTLV-1 (Table 1). No current prevalence data is available. There has been no report of the other three HTLV types.
Cabo VerdeZanella et al.65 identified cases of HTLV-1/HIV-2 co-infection in Cabo Verde. The prevalence rates of infection and co-infection were however, not defined. Full length genome characterization of the identified HTLV-1 isolates revealed them to belong to the HTLV-1aD subgroup.66 No other study is available on HTLV presence and/or prevalence in the Cabo Verdean population.
Burkina FasoThe only report on HTLV prevalence is the study conducted in 2006 by Collenberg et al.67 who also identified cases of co-infection among the study population (Table 1).
The GambiaPrevalence rates of 0–10.4% for HTLV-1 have been described in The Gambia68,69 (Table 1). HTLVs have not been reported later than 1994.
SenegalSenegal is estimated to have HTLV-1 prevalence of 143/100,000 inhabitants30,55 (Table 1). Kaplan et al.70 identified 1.5% HTLV-1 co-infection with HIV among hospital patients with the diagnosis of pulmonary tuberculosis. Seroprevalence of HTLV-2 was 0.02% among blood donors30 in Dakar. HTLV-1aD (North African subgroup) is the prevalent subgroup in Senegal. HTLV-3 and 4 have not been isolated.
TogoThe prevalence rate of HTLV-1 in Togo falls between 1.2% and 1.8%50,53,71 (Table 1). Other types of HTLV have not been reported.
Available data show that West Africa falls within regions with moderate to high HTLV-1 prevalence. Niger is the only country in West Africa with low prevalence of HTLV-1. There are few reports of HTLV-2. The co-infection of HTLV with other sexually transmitted viruses like HIV-1 or HIV-2, hepatitis viruses, and human papilloma virus (HPV) in most places is largely unknown as there are sporadic records of incidence.14 Although HTLV-3 and 4 have not been reported, there is risk of transmission of the virus types since there are reports of their presence in Cameroon, a neighbouring country.3,4
Routine screening for HTLVsThe screening of blood donors for HTLV-1/2 infection alongside other mandatory tests before donation has been mandated in many endemic countries – Asia – China, Japan, Taiwan; America: Argentina, Brazil, Canada, Colombia, French West Indies, Jamaica, Peru, USA, and Venezuela; Australia; Europe: New Zealand, Sweden, UK, Uruguay, France, Greece, Ireland, Netherlands, Portugal, Romania, Denmark, Finland, and Norway. Middle East: Israel, Iran, and Saudi Arabia.72 However, routine screening and diagnosis of HTLV-1/2 infection among blood donors in West Africa and other endemic parts of Africa, is rarely practiced, despite the fact that these regions are of moderate to high endemicity.
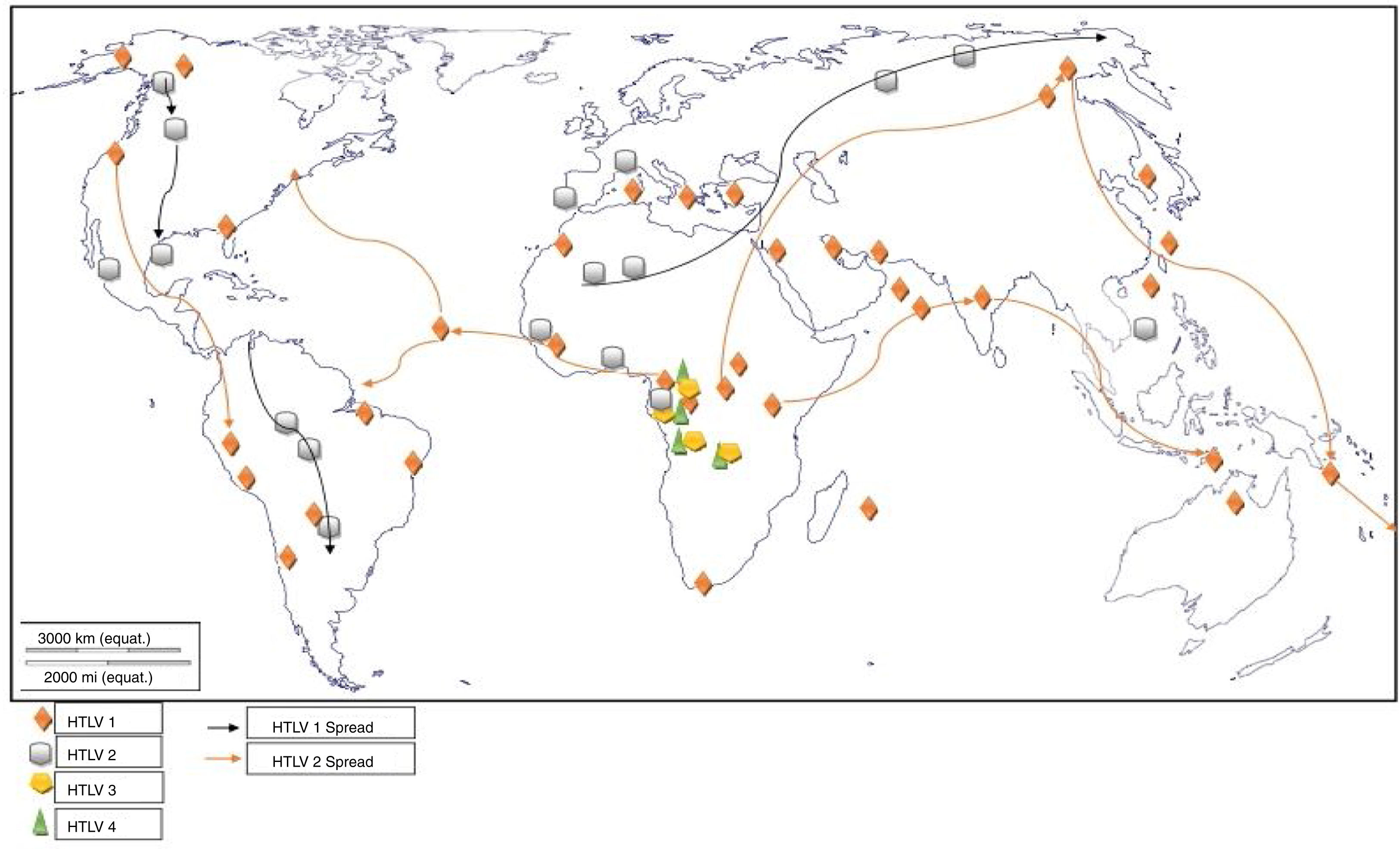
HTLV-3 and HTLV-4Two new viruses, genetically related to HTLV-1 and 2 (although more related to their STLV counterparts), were discovered in the same geographical region, the rainforest part of Southern Cameroon, Central Africa.3,4 On isolation from asymptomatic pygmies and Bantus, they were subjected to enzyme immunoassay (EIA) and Western blotting which yielded indeterminate results to both HTLV-1 and 2. Their proviruses were then detected using a series of PCR primers designed to amplify all known HTLVs and STLVs.3,4 The infected individuals were either hunters, or living in the rainforest region (Fig. 4), characterized by existence of non-human primates highly infected with STLVs. It is therefore, not out of place to suggest that they may have diverged via interspecies transmission from non-human primates to humans. The relatedness to other deltaviruses led to their placement in the same genus and family, and the designation of the names HTLV-3 and HTLV-4.
The tax gene of HTLV-3, like HTLV-1, contains a PDZ binding motif while HTLV-4 does not. The motif binds to PDZ domain and promotes virus-mediated T-cell proliferation in vitro and persistence in vivo. PDZ (postsynaptic density 95, PSD-85; Discs large, Dlg; Zonula occludens-1, ZO-1) domains are modular protein interaction domains – formerly known as Discs-large homology regions (DHRs) or GLGF repeats, after a conserved Gly-Leu-Gly-Phe sequence found within the domain – that play a role in protein targeting and protein complex assembly.73 The presence of HTLV-3 and 4 viruses in Cameroon, though not associated with any diseases, is depictive of their possible presence in other West and Central African countries.
Are indeterminate HTLV results in West Africa an insight to a new virus type?There are several reports of indeterminate WB patterns resulting from HTLV reactive sera/plasma samples in Africa. Researchers have suggested cross-reactivity with antigen of Plasmodium falciparum39 and of various infectious agents, as well as low proviral load or co-infection with other infectious agents like HIV. However, most of these indeterminate results in apparently healthy individuals are not HLTV-3 or 4, upon subjection to further confirmatory assays. Persistent increased rate of indeterminate results, which are not HTLV-3 or HTLV-4 upon subjection to further confirmatory assays, ought to be looked into. It should be recalled that HIV-1 and 2 (previously named HTLV-3 and 4) were initially grouped together with HTLV-1 and 2, and were only regrouped after further research. Therefore, further investigative research on these indeterminate cases is warranted, as there is a possibility that the indeterminate WB results encountered with EIA/PA reactive samples could be new HTLV-1, 2, 3 or 4 subtypes, yet-to-be discovered HTLV type or an entirely new undiscovered infectious agents with similar reactivity to EIA.
ConclusionAlthough the prevalence rate of HTLV in target risk populations is useful epidemiological data, they may not give a true representation for accurate estimation of HTLV infections in West Africa, as they are mostly restricted to visitations of study participants to the hospitals (hospital – based studies). Most Africans engage in self-medication, as they do not visit the hospitals until they present with severe symptoms. Giving the long latency of the virus, as well as the fact that diagnostic and/or routine screening is hitherto not mandatory, the real prevalence rates of HTLVs may be higher than those found in the reviewed studies. It should be noted that prevalence data from population-based studies have a trend to be more accurate once they are considered healthy individuals. West African lacks recent epidemiological data on HTLV prevalence. Nation-wide general population based studies capturing the communities (apparently healthy population) should therefore be considered, so as to ascertain the true prevalence of these virus types. Furthermore, the significantly high rate of HTLV indeterminate WB serological patterns in African studies calls for concern. Although cross-reactivity with antigens of some other infectious agents have been hypothesized, there are possibilities that such reactions could be resultant from yet-to-be discovered HTLV (sub)types or an entirely unknown virus/infectious agent. It is imperative that routine screening for HTLVs be mandated in West Africa, especially in the health care centers and hospitals that engage in blood donation and/or transfusion, since West Africa is a region of high endemicity of HTLV-1 and 2. It should be noted that West Africa is at risk of HTLV-3 and 4 transmission and subsequent endemicity, given the presence of the virus types in Cameroon, a neighbouring country to Nigeria, a West African country.
Conflicts of interestThe authors declare no conflicts of interest.