Herein we report a fatal case of donor-derived transmission of XDR-resistant carbapenemase-producing Klebsiella pneumoniae (KPC-Kp) in cardiac transplantation. A 59-year-old male patient with non-obstructive hypertrophic cardiomyopathy underwent heart transplantation. On day 5 post-operation, blood cultures from the donor were positive for colistin-resistant carbapenemase-producing K. pneumoniae (ColR KPC-Kp) susceptible only to amikacin. Recipient blood cultures were also positive for ColR KPC-Kp with the same sensitivity profile as the donor isolate with an identical PFGE pattern. The patient was treated with double-carbapenems and amikacin. The patient evolved to pericarditis, osteomyelitis, and pulmonary necrosis, all fragment cultures positive for the same agent. The patient developed septic shock, multiple organ failure and died on day 50 post-transplantation. Based on current microbiological scenario worldwide the possibility of transmitting multidrug resistant (MDR) organisms should be considered.
Multidrug-resistant Gram-negative bacterial infections in solid organ transplantation have been increasingly recognized in the past decade.1 Most recently, XDR-resistant carbapenemase-producing Klebsiella pneumoniae (KPC-Kp) infection has emerged as a significant healthcare challenge, especially considering that few antibiotics are effective to treat it.1 The incidence of donor-derived transmission of infections is <1% in solid organ transplants (SOTs), with morbidity and mortality rates of 40%.2 This case report aims to address the challenges involved in prevention, detection and management of donor-derived infection by MDR organisms.
Case reportA 59-year-old male patient with non-obstructive hypertrophic cardiomyopathy was hospitalized to manage cardiac insufficiency with inotropic therapy. The worsening of his clinical picture required the use of an intra-aortic balloon, and he was prioritized for cardiac transplantation. During the pre-transplant period, the patient did not develop any infectious complications. Blood cultures collected 24 and 48h before transplantation were negative.
The patient underwent bicaval–bipulmonary heart transplantation, which required another surgery in the first 24h to resolve hemostasis. He remained under mechanical ventilation with the use of vasoactive drugs and an intra-aortic balloon for 48h, maintaining a good clinical condition. The immunosuppressive regimen included mycophenolate mofetil, cyclosporine and prednisone. On day 3 post-operation, a blood culture from the donor was positive for Gram-negative bacilli. As the donor was receiving piperacillin–tazobactam at the time of transplantation, the same drug was administered to the recipient. Although the recipient was clinically stable without signs of infection, blood cultures and surveillance cultures (from the groin and rectal areas) were collected. On day 5 post-operation, the final identification turned out ColR KPC-Kp.
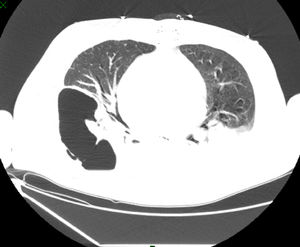
Blood cultures from the recipient were positive for ColR KPC-Kp on day 7 post-operation with the same sensitivity profile as the donor isolate. The surveillance cultures were negative. At this time, the recipient was afebrile and hemodynamically stable, presenting with 26,000cells/mm3 leukocyte count and 6.5mg/dL C-reactive protein (CRP) levels. Double-carbapenems (meropenem 2g 8/8h in a 4-h infusion combined with ertapenem 1g/day, 1h before one of the meropenem doses) and amikacin (15mg/kg once a day) was started. The patient developed pericarditis on day 9 post-operation and required the drainage of 1000mL of exudate; his pericardial fluid cultures were positive for ColR KPC-Kp. The patient underwent a surgical approach to clean the pericardium, requiring a repeated surgery eight days later; a sternum bone fragment collected at this time was positive for ColR KPC-Kp. On day 37 post-operation, he presented with worsening respiratory signs with chest computed tomography exhibiting consolidation with lung fluid levels suggestive of pulmonary cavitation due to necrosis (Fig. 1). This diagnosis was confirmed by histopathology of lung fragments obtained from lobectomy, in which a pulmonary abscess with liquefactive necrosis and necrotizing arteritis were noted. The patient developed septic shock, and antibiotic coverage was extended to linezolid and fluconazole while maintaining the initial treatment regimen for ColR KPC-Kp (double-carbapenem and amikacin). The patient progressed to multiple organ failure and died on day 50 post-transplantation.
The donor and other recipientsThe donor was a 17-year-old male who died from traumatic brain injury after being hospitalized in the intensive care unit (ICU) for seven days having received piperacillin/tazobactam and vancomycin for two days. His blood cultures and surveillance cultures were negative at the time of donation. The culture that was positive for ColR KPC-Kp was collected from the splenic artery at the time of organ removal. The recipients of other organs (two kidneys) did not present any infectious complications or positive cultures and did not receive specific antimicrobial treatment. The liver was not used for transplantation.
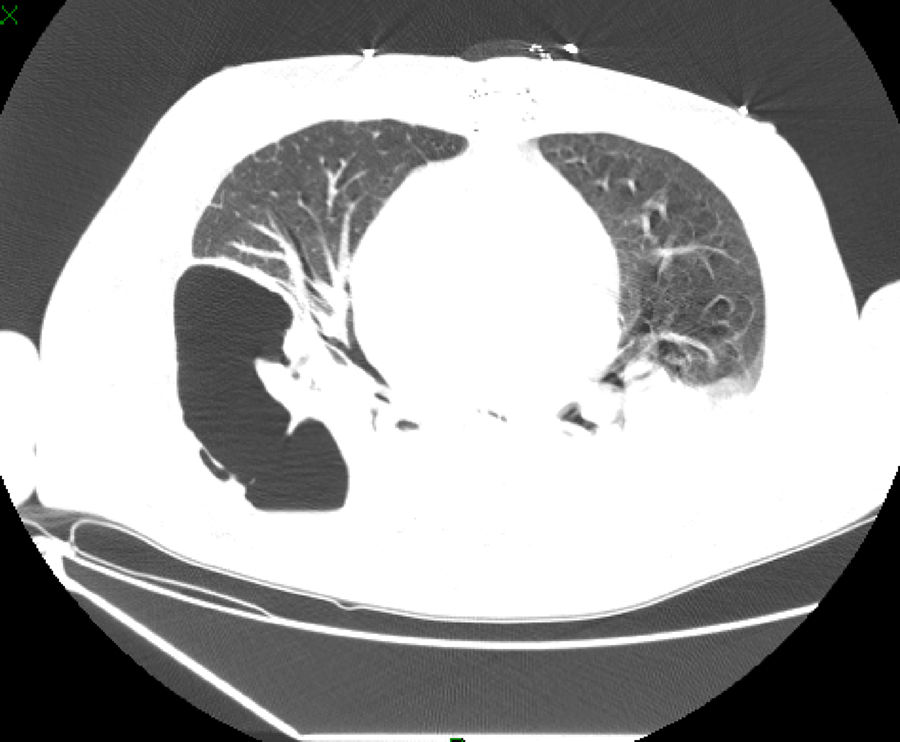
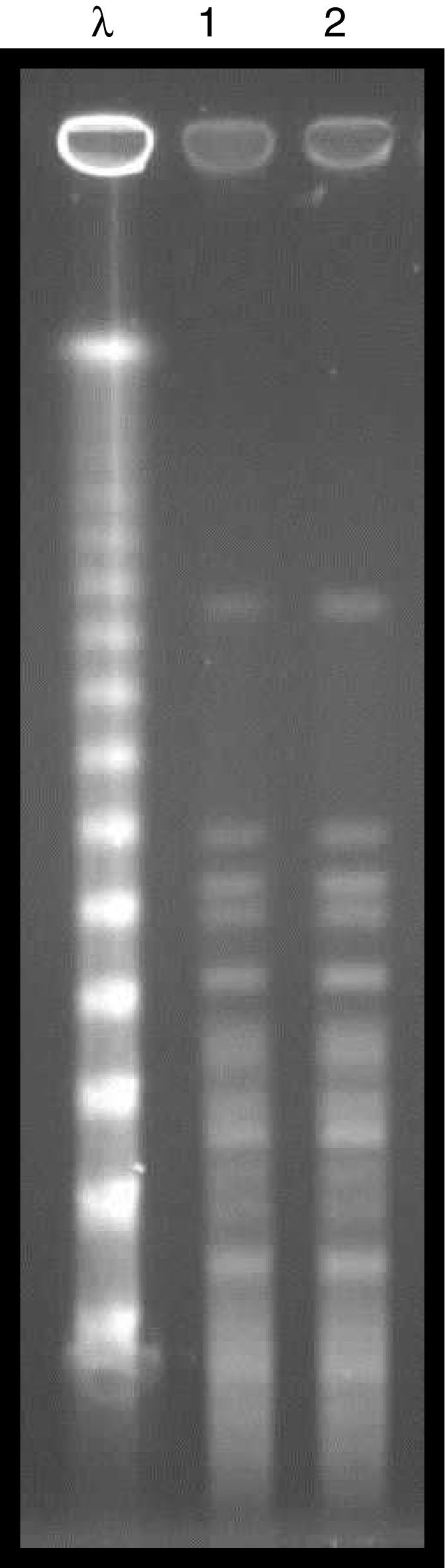
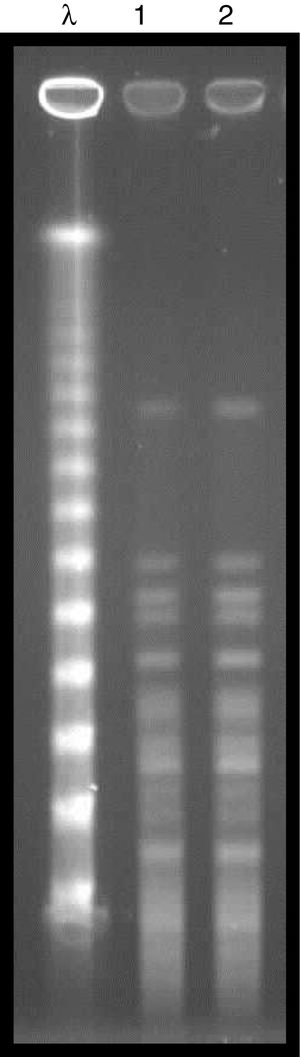
Microbiological aspectsBacterial identification was performed by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF/MS) technology, using Vitek MS® system (bioMerieux, Marcy-I’Étoile, France), and the antimicrobial susceptibility profile was determined using the Vitek® 2 system (bioMerieux). The minimum inhibitory concentration (MIC) for polymyxin B was defined by broth microdilution (Probac, Brazil). The two strains evaluated (donor and recipient) exhibited high levels of resistance to cefepime, cefoxitin, ceftazidime, ceftriaxone (MIC≥64μg/mL), ertapenem (MIC≥8μg/mL), imipenem (MIC, 8μg/mL), meropenem (MIC≥16μg/mL), ciprofloxacin (MIC, ≥4μg/mL), gentamicin (MIC≥16μg/mL), and tigecycline (MIC, 4μg/mL) following the breakpoints established by CLSI, 2016.3 Resistance to polymyxin B (MIC, >64μg/mL) was established using the EUCAST breakpoint, 2017.4 Amikacin was the only susceptible drug (MIC, 4μg/mL). The presence of the blaKPC resistance gene was detected in all strains evaluated,5 and the molecular gene identification using Sanger sequencing exhibited 100% homology with the enzyme KPC-2. The genetic similarity between strains was established by pulsed field gel electrophoresis (PFGE) using the SpeI restriction endonuclease, demonstrating an identical PFGE pattern (Pattern A) between the donor and recipient strain (Fig. 2). Multilocus sequence typing (MLST) analysis also identified the same sequence type (ST 437) from the donor and the recipient strains, and eBURST analysis (http://eburst.mlst.net) revealed that ST 437 belonged to clonal complex CC 258.
DiscussionTo date, this is the first report of donor-derived transmission of XDR-resistant KPC-Kp in cardiac transplantation. Based on the categorization suggested by Garzoni and Ison, this case reports a proven transmission of infection by a donor with a fatal outcome.6 The strains isolated from the recipient and donor exhibited a unique PFGE pattern and the same sequence type (ST 437). Both strains were isolated and identified early in the post-transplant period without any clinical or microbiological evidence of infection in the recipient during the pre-transplant period. The agent was identified using traditional culturing methods following organ acceptance, with no possibilities for directed prophylaxis and adequate early treatment that could have contributed to a favorable outcome.2
The incidence of donor-derived transmission of infections is <1% in solid organ transplants (SOTs), with morbidity and mortality rates of 40%.3 There are infection screening protocols for donors to reduce the risk of transmission. Requesting blood and urine cultures from donors hospitalized for more than 72h is suggested.2 However, with the increased prevalence of multidrug-resistant organisms (MDROs) the transmission of MDROs has increased.1 The amount of time required by traditional sample collection methods to the final results, including the susceptibility profile, can range from 48 to 120h, depending on detection time and the need for additional resistance testing. This delay is a matter of concern, affecting the time of introduction of proper antimicrobial therapy. Studies demonstrate that the mortality rate for KPC infections in SOTs is between 40 and 71%.7 Therefore, surveillance swab samples and cultures (blood cultures, urine cultures and tracheal secretions) are suggested for the rapid identification of resistance genes in hospitals with an epidemiological history of these agents.8 Communication delays are another contributing factor for the transmission of infection,9 fast and effective communication between Organ Procurement Organizations (OPOs) and transplant centers is highly important and should occur within 24h.
Due to organ shortages, the use of donor organs known to be infected has been discussed. Accepting more borderline donors has been proposed, including those with bacteremia. Data indicate that 5% of donors have bacteremia at the time of donation; fever in the last 24h is predictive of this situation.10 Studies have demonstrated that bacteremia due to Gram-negative bacilli has an increased risk of transmission and worse outcomes compared with Gram-positive bacteria.11 Ideally, the donor should already be on appropriate treatment for at least 24–48h prior to donation with some degree of clinical response, and treatment for the recipient should be maintained for 7–14 days.8,12 However, the use of donor organs with multidrug resistant (MDR) bacterial infections is a major problem. Studies have proposed the donation of all organs from colonized donors and the use of prophylaxis directed at the recipient. These studies suggest that lungs and kidneys should be rejected if tracheal secretion and urinary cultures are positive, and all organs should be rejected if the blood cultures are positive.8,13 However, a risk-benefit analysis should always be performed that also considers the risk of death of those on the transplant waiting list.8,12 Ariza-Heredia et al. reported on four recipients from a donor with KPC-Kp meningitis but negative blood cultures. The recipients received adequate prophylaxis and exhibited good progression after transplantation. The authors demonstrated the possibility of using organs from donors with MDR infections with rapid communication between centers to allow for preventive measures and adequate treatment for the recipients.14
It is intriguing that kidney recipients did not develop infection. Berenger et al. studied the risk factors for nosocomial bloodstream infections in SOT and found a reduced incidence in kidney compared to heart recipients (0.54 vs. 1.5/100 patient-years).15 However, Yeşilkaya et al. reported similar rates of bloodstream infections in heart and kidney transplantation patients.16 The reasons behind this finding are complex and not fully understood yet.
Our patient progressed with hematogenous dissemination of ColR KPC-Kp; he displayed pericardial and bone (sternal) involvement and necrotizing pneumonia. However, he did not initially exhibit signs and symptoms of infection, likely due to immunosuppression. Treatment with double-carbapenems and amikacin (the only susceptible drug in the antibiogram) was not effective in controlling the spread of infection probably because of the low effectiveness of the regimen and late treatment initiation, since the donor was not known to be infected.17 The choice of antimicrobial regimen was based on the lack of therapeutic options for the treatment of ColR KPC-Kp given that new therapeutic options, such as ceftazidime–avibactam and meropenem–vaborbactam, are not available in Brazil. This regimen was successfully used in one kidney transplantation despite meropenem MIC≥16μg/mL.18 Infection/colonization by ColR KPC-Kp susceptible only to amikacin should be a relevant factor when considering organ acceptability. A 2015 study reported a 51% mortality rate at 30 days for ColR KPC-Kp bloodstream infections.19
ConclusionDespite screening policies, the possibility of donor-derived infection exists. This case brings up many challenges that contributed to a fatal outcome such as gaps in screening tools, delay in laboratory identification by traditional culture methods, lack of therapeutic options and associated immunosuppression.
Conflicts of interestThe authors declare no conflicts of interest.