A/H1N1 influenza is a viral disease that affects a significant part of the population mainly in winter, leading to increased number of medical consultations, hospitalizations and consequently care spending in emergency.
MethodsThis is a case-series retrospective study, involving patients admitted to a tertiary hospital in southern Brazil in 2016 with a clinical diagnosis of acute respiratory infection of the influenza type and laboratory confirmation of influenza A/H1N1.
Results64 patients were included, mostly male, median age of 48.3 months. Chronic underlying diseases were found in 73% of the patients, and these patients evolved to the most unfavorable outcome. About vaccination, of the 57 patients with an age range for vaccination, only 28% had complete vaccination coverage. The main clinical manifestations found in the included patients were fever, cough, intercostal indrawing, wheezing, tachypnea and pulmonary crackles. These patients were mainly followed-up with laboratory tests and chest X-ray. Consolidation was evident in 43% of patients followed by interstitial infiltrate in 33%. A five-day course of neuraminidase inhibitor was prescribed for all patients, as recommended by the WHO, but due to the complications, 73% of the patients required antibiotic therapy, and 61% oxygen therapy. The majority of patients had a favorable outcome, but 11 required intensive care and one died.
ConclusionsA/H1N1 influenza persists as an important public health problem, mainly due to high morbidity and hospitalization rates. It is important to identify patients with A/H1N1 influenza and clinical situations with higher risk of complications. Through this study, it is possible to analyze the characteristics of pediatric patients with A/H1N1 influenza and mainly to emphasize assistance of populations with comorbidities, since they present higher rates of complications and death.
Influenza A/H1N1 is an acute infectious disease with great morbidity, capable of impacting the routine of individuals, such as absenteeism at school and at work, increasing the number of visits to medical care units, resulting in higher cost of care and overcrowding hospitals. In children, the outcome of the disease tends to be less favorable. In addition, children are important vectors for spreading the disease.1–4
In Brazil, in 2016, even before the winter months, several cases of A/H1N1 influenza were diagnosed, specially in the adult population. The legacy of the 2009 pandemic was essential for the establishment of diagnostic protocols and clinical management, avoiding panic amongst the populations involved.
The objective of this study was to analyze a case-series of pediatric patients admitted to Hospital de Clínicas de Porto Alegre (HCPA) in 2016 with A/H1N1 influenza, taking into account demographic characteristics, risk factors, clinical and laboratory manifestations, length of hospitalization, therapy used, and disease outcome.
MethodsHCPA is a public teaching hospital of the Federal University of Rio Grande do Sul (UFRGS), Brazil. It is an 842-bed tertiary care facility with an average of 30,000 admissions/year. During 2016 all pediatric patients admitted to HCPA with a clinical diagnosis of influenza-like acute respiratory infection with laboratory confirmation of influenza A/H1N1 virus by RT-PCR (polymerase chain reaction) collected from the nasopharynx were included in the study.1,5,6 This was a case-series retrospective study with data abstracted from patients’ records.
The eligibility criteria were: (a) age between 1 month and 14 years; (b) clinical diagnosis of A influenza according to criteria of the World Health Organization (WHO), Centers for Disease Control and Prevention (CDC), and the Brazilian Ministry of Health: “fever with sudden onset, even if reported, accompanied by cough or sore throat and at least one of these symptoms – myalgia, headache or arthralgia. In children less than two years of age, with no other specific diagnosis: sudden onset of fever, even if reported, and respiratory symptoms – cough, coryza and nasal obstruction; and (c) laboratory confirmation of A/H1N1 influenza by RT-PCR in a sample collected with a nasopharyngeal swab”.1,5,6
Personal and family history, demographical, epidemiological, clinical and laboratory data, and disease outcome of included patients were recorded and stored in a database.
Continuous variables are expressed in absolute values and percentages, together with mean, median, and standard deviation. Pearson's chi-square test was used to compare the categorical variables or Fisher's exact test for variables with expected values lower than 5. Variables with non-normal distribution were compared with the Mann–Whitney test. The value of p<0.05 was considered statistically significant. IBM Statistical Package for the Social Sciences (IBM SPSS Statistics 17.0) was used for all statistical analyses.
This study was approved by the Research Ethics Committee of the HCPA, under the registration number CAAE 62915716.5.0000.532.
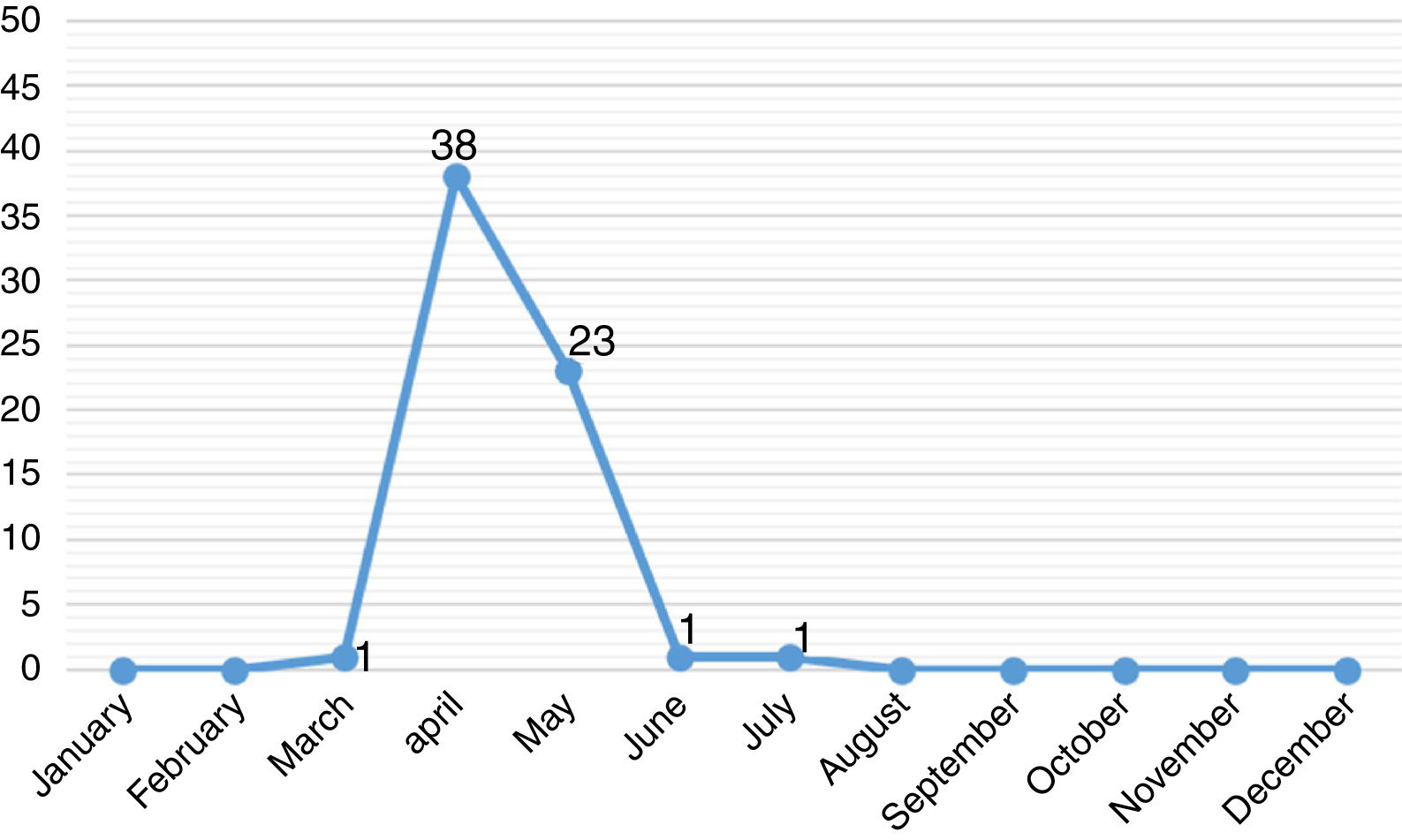
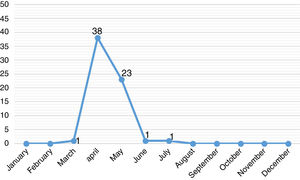
ResultsThe study included 64 pediatric patients with diagnosis of A/H1N1 influenza confirmed by RT-PCR. The majority of these patients were male (66%) and residing Porto Alegre (58%). The median age was 48.3 months, 20 (31%) were less than one year old. The monthly distribution of these patients may be observed in Fig. 1.
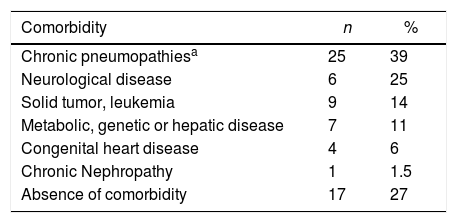
Out of the 64 included patients, 42 (66%) had their diagnosis performed at the Pediatric Emergency Unit (PEU), 17 (27%) at the Pediatric Inpatient Unit (PIU), three (5%) at the Pediatric Intensive Care Unit (PICU) and two (3%) at Pediatric Oncology Unit (POU). Of those, 47 (73%) patients had one or more pre-existing chronic underlying disease, including pneumopathies (asthma, cystic fibrosis, obliterans bronchiolitis), neuropathies, immunosuppression, or metabolic and genetic diseases, as seen in Table 1. Several patients had an association of two or more chronic conditions.
Underlying chronic diseases in pediatric patients with A/H1N1 influenza at HCPA in 2016.
| Comorbidity | n | % |
|---|---|---|
| Chronic pneumopathiesa | 25 | 39 |
| Neurological disease | 6 | 25 |
| Solid tumor, leukemia | 9 | 14 |
| Metabolic, genetic or hepatic disease | 7 | 11 |
| Congenital heart disease | 4 | 6 |
| Chronic Nephropathy | 1 | 1.5 |
| Absence of comorbidity | 17 | 27 |
Overall, seven (11%) individuals were less than six months of age, therefore within an age group with no indication for influenza A vaccination. Of the remaining 57 patients, only 16 (28%) had been vaccinated.
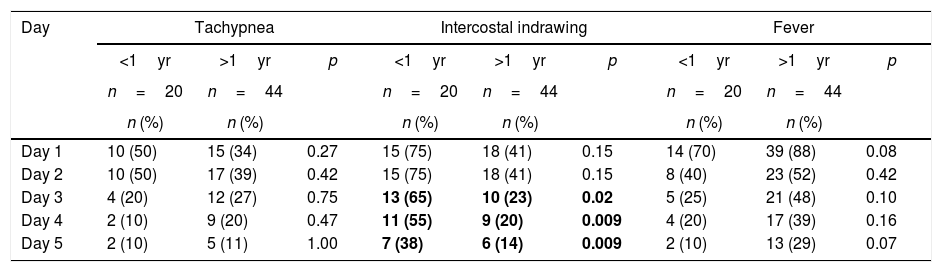
The main clinical manifestations were fever (83%), cough (73%), intercostal indrawing (52%), wheezing (41%), tachypnea (39%) and pulmonary stertor crackles (39%). No gastrointestinal manifestations were present in the initial clinical examination of these patients. Most of the signs and symptoms became less frequent from the third day on, regardless of the patient's age. Particularly, it should be noted that the presence of intercostal indrawing was more significant in children less than one year old also from the third day of hospitalization (65% vs. 23%, p<0.05), according to Table 2. The mean interval between onset of symptoms suggestive of influenza A and collection of respiratory secretion for viral PCR was 1.6 days.
Clinical manifestations in pediatric patients with A/H1N1 influenza in HCPA at 2016 according to disease progression and age group (n=64).
| Day | Tachypnea | Intercostal indrawing | Fever | ||||||
|---|---|---|---|---|---|---|---|---|---|
| <1yr | >1yr | p | <1yr | >1yr | p | <1yr | >1yr | p | |
| n=20 | n=44 | n=20 | n=44 | n=20 | n=44 | ||||
| n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | ||||
| Day 1 | 10 (50) | 15 (34) | 0.27 | 15 (75) | 18 (41) | 0.15 | 14 (70) | 39 (88) | 0.08 |
| Day 2 | 10 (50) | 17 (39) | 0.42 | 15 (75) | 18 (41) | 0.15 | 8 (40) | 23 (52) | 0.42 |
| Day 3 | 4 (20) | 12 (27) | 0.75 | 13 (65) | 10 (23) | 0.02 | 5 (25) | 21 (48) | 0.10 |
| Day 4 | 2 (10) | 9 (20) | 0.47 | 11 (55) | 9 (20) | 0.009 | 4 (20) | 17 (39) | 0.16 |
| Day 5 | 2 (10) | 5 (11) | 1.00 | 7 (38) | 6 (14) | 0.009 | 2 (10) | 13 (29) | 0.07 |
Overall, 96 chest radiographs and 68 hemograms (blood count) and biochemical studies were requested. At hospital admission, pulmonary radiographs were requested for 52 (81.2%) patients. The main radiological findings on this first day of hospitalization were pulmonary consolidation in 27 (43%), interstitial infiltrate in 21 (33%), or normal pattern in 12 (19%).
All patients were orally treated with neuraminidase inhibitor (oseltamivir) for five days, as recommended by the WHO and the Ministry of Health. A total of 47 patients received antibiotic therapy (73%), but only 10 (16% of the total) started that treatment on the first day of hospitalization. Oxygen therapy was required in 39 (61%) patients.
Regarding clinical evolution, of the 42 patients diagnosed with influenza A/H1N1 in the Pediatric Emergency Unit (PEU), 17 (27% of the total) were discharged after a mean hospital stay of 1.1 days. On the other hand, 24 patients were transferred to Pediatric Admission Unit (PAU) and had a mean hospital stay of 9.5 days (p<0.05). All the 17 (27%) patients who had the diagnosis of A/H1N1 influenza performed at PAU were discharged after average hospital stay of 11.6 days. Of all the patients studied, 11 (17%) needed PICU care, two of whom were transferred from another hospital. They all had an underlying chronic illness. Only one (2%) patient died (with chronic liver disease). Patients with A/H1N1 flu tied up a total of 506 hospital bed-days.
DiscussionIn April 2009, the CDC identified pandemic influenza A/H1N1 (2009) virus and in July, WHO declared a new pandemic caused by this viral subtype. Worldwide, during the pandemic phase, the finding of sustained transmission of this virus resulted in changes in the investigation and management of cases of influenza-like illness. In the following years the circulation of A/H1N1 virus continued to be observed along with other seasonal viruses.7–9 In 2016, at HCPA, cases of A/H1N1 influenza in pediatric patients were diagnosed at the end of March and the main hospitalization period was between April and May, months before winter. Similarly, anticipation of positive cases of A/H1N1 influenza was seen in February. Although A influenza occurs during the whole year, it is mainly prevalent in the cold months.7,8,10 When the first cases arises, A influenza spreads rapidly. Considering the pediatric population, the incubation period of the influenza virus varies from one to four days and the transmissibility lasts, on average, for 10 days, but can be prolonged in immunosuppressed patients.7–16
In the current study, the majority of cases were male residing in Porto Alegre metropolitan area with similar demographic characteristics of other groups surveyed.1,8,11 Patients with A/H1N1 influenza and underlying chronic illnesses, besides having a potential for increased transmissibility of the disease, are also thought to have greater morbidity, especially severe pneumonia, and mortality. In this study, 73% of the patients presented some comorbidity. Studies of pediatric patients with A/H1N1 influenza in 2009 reported about one-third (32 and 35%) of pre-existing clinical conditions, including asthma, immunosuppression, chronic lung disease, and neurological disorder.1,7,11,17,18
Worldwide, vaccination against A/H1N1 influenza is recommended for all infants between six and 24 months, including those previously healthy, to provide protection against clinical complications of the disease, and to reduce the spread of the virus, morbidity and mortality, including in elderly groups. Even so, vaccination coverage remains very low in several countries.2,3,8,11,19–22 Vaccination coverage against A/H1N1 influenza in the present study was of only 28%.
The main clinical manifestations of patients with A/H1N1 influenza in this study were fever, cough, intercostal indrawing, wheezing, tachypnea and pulmonary crackling stertors. Those clinical signs decreased in frequency after the third day of disease progression. As the observed clinical manifestations of mild cases are very similar to those seen in seasonal influenza and in other viral respiratory diseases, the diagnostic delay is common. The most serious situations, however, are accompanied by fever, cough, shortness of breath, fatigue or weakness, chills, myalgia, rhinorrhea, sore throat, headache, wheezing, vomiting, and diarrhea. The present study did not find a relationship between symptoms and age group, but highlighted the significantly greater presence of pulmonary stertors in children less than one year of age, especially after the third day of hospitalization. Some studies highlight the importance of gastrointestinal symptoms, especially in the infant population (25–38%); however, these manifestations were absent in our sample.6,7,9,20,23–27
For the patients evaluated in this study, the most requested exams were hemogram (blood count) and pulmonary radiographs (19% were normal; no change in management). Respiratory secretion was collected for detection of H1N1 by RT-PCR in all patients. This method is fast, accurate and, despite the high cost, has been indicated as the gold standard for H1N1 diagnosis. At HCPA, the result of A/H1N1 influenza virus by RT-CR turned out within 24–48h. Rapid confirmation of diagnostic results in more adequate clinical management: therapy reinforcement or discontinuation, orientation to family members, and transfer to another hospitalization unit or hospital discharge.28–30
In our study, all patients received oseltamivir. Some authors suggest that this drug, when used at the beginning of the infectious process in patients with more severe symptoms, may be able to improve disease outcome and, consequently, its potential complications. Since the pandemic in 2009, oseltamivir has been widely prescribed for the treatment of A/H1N1 influenza, but the evidence on the effectiveness of the drug is controversial.7,16,27,31
A high number of patients received antibiotic therapy (73%), which is reported by several authors due to the frequency of associated bronchopneumonia, but only 10 (16%) started this treatment on the first day of hospitalization. Oxygen therapy was required in only 39 (61%) patients. There have been studies demonstrating that oxygen supplementation was required in more than 80% of pediatric patients hospitalized with A/H1N1 influenza, during an average of six days of use.1,7,16,27
Only one hospitalized patient died. Hospitalized patients were a group of high morbidity and clinical severity, since they had more underlying chronic disease (73%), low vaccine coverage of A influenza (28%), developed pneumonia as a complication (47%), and required more often oxygen (61%) and antibiotic therapy (73%). The main clinical complications of patients with A/H1N1 influenza were otitis, pneumonias, meningitis, myositis and myocarditis. These findings are quite similar to those reported by other authors who analyzed pediatric populations with A/H1N1 influenza, especially in the 2009 pandemic.1,2,4,7,9,13–15,17,24,32–37
Despite the large number of studies on A/H1N1 influenza each year, this disease persists as an important public health problem, leading to alertness, mainly due to the high morbidity and need for hospitalization. It is important to identify, as early as possible, patients and clinical situations with higher risk of developing complications. In this case-series of pediatric A/H1N1 influenza most of the children studied had comorbid conditions, but nonetheless disease outcome of these children with higher risk was favorable, with only one (2%) death. Among the legacies of the 2009 pandemic, the most outstanding were strategies for disease control and care for patients considered at risk, which probably explains our results.1,7,22–24
Conflicts of interestThe authors declare no conflicts of interest.