The long-term consequences of COVID-19, especially pulmonary impairment, are frequent but not well understood. The knowledge about sequels or long COVID-19 are necessary, considering the high prevalence and need for specific public strategies.
MethodThe study was conducted to evaluate symptoms (standardized questionnaire), pulmonary function (spirometry), and exercise capacity (6-minute-walk-test) at 30 (D30), 90 (D90), and 180 (D180) days after hospital discharge of patients surviving to severe COVID-19. We excluded in this follow up patients with comorbidities before COVID infection.
Results44 patients were included and 31 (26 men) completed the 6-month follow-up (age mean 53.6 ± 9.6 years). At D180, 28% presented still at least one symptom. The most common was dyspnea (17.2%), followed by cough (13.8%), and myalgia (10.3%). All spirometric parameters showed progressive improvement from D30 to D180. However, 16% maintained a restrictive pattern on spirometry test, 44% presented desaturation on the 6-minute walk-test, and 25% walked < 75% of the predicted value.
Conclusion6-months after hospital discharge, reduced pulmonary function and reduced exercise capacity was founded frequently and more than a quarter remained symptomatic. The persistent symptoms and functional impairment suggest that sequels and development of Long COVID-19 are very common. The identification of these patients to provide the necessary health care is a challenging task, considering the large number of patients infected and surviving to COVID-19 disease.
Since November 2019, the emerging infectious disease caused by Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2), named Coronavirus Disease 2019 (COVID-19), has spread worldwide, resulting in more than 486 million confirmed cases and more than 6.1 million deaths by April 2022.1 The clinical manifestations of COVID-19 broadly range from mild to severe presentations. Most of the patients show symptoms remission soon after cure of the acute infection. Others, however, relate symptom's for more than one year, suggesting what has been denominated as 'long COVID syndrome'. defined as health impairments experienced after > 4 weeks of SARS-CoV-2 infection remission.2 These patients usually relate not only respiratory symptoms such as dyspnea and cough, but also systemic claims as fatigue, neurological and psychiatric disturbances. The mechanisms of these extra-pulmonary and chronic manifestations remain unclear.
The pulmonary lesions refer to direct consequences of virus and inflammation in the lung, resulting in diffuse alveolar and capillary damage, hyaline membrane formation, alveolar septal fibrous proliferation, and pulmonary consolidation. Reduced diffusion capacity for carbon monoxide (DLCO), impairment of exercise capacity, and altered spirometry parameters, have been described, especially in severe cases of COVID-19. The time-course and the extent of this recovery, however, are poorly studied.3
Survivors to severe acute respiratory syndrome and to the Middle East respiratory syndrome have reduced lung function for up to 6-months following hospital discharge.4,5 However, there are few long-time follow-up studies of patients after severe COVID-19.6-8 Most of the studies included patients with previous comorbidities9-12 what difficult to identify lesions and disabilities related exclusively to COVID-19 or those related to previous conditions. The long-term consequences are not completely understood but is necessary to evaluate objective parameters to establish the late repercussions of COVID-19 on pulmonary function and its impact on functional capacity and quality of life.
In this study our aim was to follow-up survivors of severe COVID-19 without previous comorbidities in order to identify subjects with persistent pulmonary impairment after hospital discharge due to COVID-19 infection.
Materials and methodsThe study was conducted at the University Hospital of Federal University of Espírito Santo, Brazil according to a protocol previously approved by the Institutional Ethics Committee (n° 3,503,292,000,005,071). All participants signed a consent form before enrollment. Inclusion criteria were age 18‒70 years, confirmed diagnosis of COVID-19 by positive RT-PCR or serologic test for SARS-CoV-2, and presentation of the severe form of the disease. The severe cases were defined as: 1) Severe pneumonia-fever or suspected respiratory infection, plus one of the following criteria: respiratory rate > 30 min, severe respiratory distress, or SpO2 ≤ 93% at rest and on room air, 2) Acute Respiratory Distress Syndrome (ARDS).13
Patients who developed extrapulmonary manifestations of COVID-19 during hospitalization or with lack of data were excluded. For the present analysis we excluded patients with previous diseases such as heart failure, pneumopathies (i.e., asthma and chronic obstructive pulmonary disease), nephropathy, psychiatric, or neurologic disorders and those with severe obesity (Body Mass Index ‒ BMI ≥ 4 kg/m2).
After hospital discharge, patients were invited to attend a medical evaluation program at 30 (D30), 90 (D90), and 180 (D180) days after hospital discharge. On D30, a standardized questionnaire was used to define the demographic profile of participants. Duration of each symptom was monitored using a standardized questionnaire containing yes/no questions. Spirometry and 6-Minute-Walk Test (6-MWT) were performed at each assessment (D30, D90, and D180). For pulmonary evaluation, patients underwent spirometry (Koko Sx, Philips, USA) to measure the Forced Expiratory Volume in the 1st second (FEV1) and Forced Vital Capacity (FVC). The standardization and categorization of functional changes were based on Brazilian guidelines for pulmonary function tests.14 FVC < 80% of the Predicted Value (PV) was considered as pulmonary dysfunction, which was classified as mild (FVC 60%‒80% PV), moderate (FVC 50%‒59% of PV), or severe (FVC < 50% of PV).
Exercise capacity was evaluated using the 6-MWT. This was performed using a JG MORIYA model 1001 pulse oximeter. The following parameters were measured: (1) Basal Oxygen Saturation (SpO2) at rest, (2) Oxygen desaturation during exercise (defined as a decrease > 3% in relation to the basal SpO2), 3) The lowest SpO2 measured during the exercise (nadir SpO2), and (4) The 6-min Walk-Distance (WD) which was considered reduced when < 75% of the PV.15 The PV of the WD in the 6-MWT was calculated by the formula: WD(m)=(7.57×heightcm)−(5.02×age)−(1.76×weightkg)−309mformen;andWD(m)=(2.11×heightcm)−(2,29×weightkg)−(5.78×age)+667mforwomen16
Statistical analysisCategorical data were presented as numbers of individuals and percentages. Quantitative analyses were presented as observed frequency, percentage, central tendency, and variability. The normal distribution of the sample was verified using the Shapiro–Wilk test. Analysis of variance for repeated measures (ANOVA) followed by the Bonferroni post hoc test was used to evaluate the evolution of FVC and FEV1 in spirometry and the WD in the 6-MWT. The Greenhouse-Geisser sphericity correction was applied to the WD analysis. ANOVA and the post-hoc Dwass-Steel-Critchlow-Fligner pairwise comparisons test were used to compare the nadir SpO2 in the 6-MWT. Categorical data were analyzed using the chi-squared test and Fisher's exact test. An alpha level of 5% significance was used for all analyses. Data were analyzed using Jamovi statistical software (version 1.8).
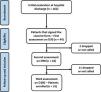
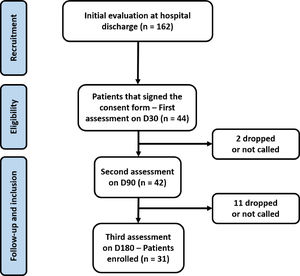
ResultsA total of 162 patients were approached in the initial evaluation at hospital discharge and 94 attended to the outpatient clinic to the first evaluation (D30). However, only 44 attended to all criteria of inclusion and exclusion and completed the initial exams. Other 13 patients were lost during the follow up period and only 31 completed all the follow up period (Fig. 1) and were included in the present analysis.
The age mean was 53.6 ± 9.6 years old, and 22 (71%) participants were male. During hospitalization, 17 (54.8%) used ivermectin, and 5 (16.1%) used hydroxychloroquine. During hospitalization, all patients received antibiotic therapy and approximately one-third were treated with corticosteroids. Intensive care unit admission was required for 54.8% of the patients. The demographic profile and hospitalization data of the cohort is shown in Table 1.
General characteristics of the 31 patients followed up for 180 days after hospital discharge after COVID-19.
| Age (years) | 53.6 ± 9.6 |
|---|---|
| Sex ‒ n (%) | |
| Men | 22 (71) |
| Women | 9 (29) |
| Previous comorbidities ‒ n (%) | |
| Arterial hypertension | 15 (48.4) |
| Diabetes mellitus (type 2) | 11 (35.5) |
| Overweight (BMI 25‒29.9 kg/m2) | 14 (45.2) |
| Obesity (BMI 30‒40 kg/m2) | 10 (32.3) |
| Smoking ‒ n (%) | |
| Current | 0 (0) |
| Past | 5 (16.1) |
| Never | 26 (83.9) |
| Sedentary lifestyle ‒ n (%) | 21 (67.7) |
| Hospital stay ‒ n (%) | |
| Pre-hospitalization symptomatic period (days)a | 10.7 ± 3.4 |
| Hospitalization period (days) | 8.8 ± 5.9 |
| Need for ICU | 17 (54.8) |
| ICU days | 17 ± 5.4 |
| Need for intubation | 3 (9.7) |
| Pulmonary thromboembolic event | 6 (19.4) |
Data are reported as mean ± standard deviation or as number and (percentage).
BMI, Body Mass Index; ICU, Intensive Care Unit.
The most common initial symptoms in acute phase were fever (80%), cough (86.7%) and dyspnea (76.7%). The first assessment (D30) was performed 31 to 36 days after hospital discharge and almost all patients were fever-free. The most persistent symptoms at D30 were dyspnea (80%) and cough (50%). In D90, almost half of the cohort presented 5 or more symptoms and referred difficulties to return to their habitual work. At D180, 28% still persisted with at least one symptom being the most frequents dyspnea (17.2%), cough (13.8%), and myalgia (10.3%). The initial presentation of the disease and evolution of symptoms in the post-discharge assessments are presented in Table 2. The radiological evaluation with chest High Resolution Tomography (HRCT) showed pulmonary involvement from 26%‒75% in 95% of the patients.
Clinical manifestations before hospitalization and at different times after hospital discharge of patients after COVID-19 (n = 31).
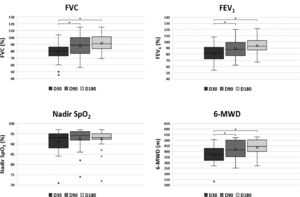
All parameters assessed by spirometry and 6-MWT showed progressive improvement and are shown in Fig. 2 and Table 3. The FVC, FEV1 and the WD in the 6-MWD values increased progressively during the observational period, mostly in the first 90 days after hospital discharge (p = 0.001 and p = 0.003, respectively). At D30, 14 (45%) patients showed a restrictive pattern on spirometry and at D180, 5 (16%) patients persisted with a FVC below the predicted value suggesting a restrictive pattern, The WD improved in all patients, but at D180, 2 (6%) patients still presented a WD < 330 m and in 8 (25%) patients it was < 75% of the PV. The results of exercise capacity are shown in Table 3. A total of 13 (44%) patients showed desaturation at D180. On D180, the correlation between FVC, SpO2 Nadir, and WD showed no statistical significance in any of the associations.
Boxplot of pulmonary function parameters at 30 (D30), 90 (D90) and 18 (D180) after hospital discharge of patients (n = 31) with severe COVID-19. Upper left: FVC, Forced Vital capacity; Upper right: FEV1, Forced Expiratory Volume in 1 second; Lower left: Nadir SpO2; Lower right: 6-MWD, 6-Minute Walked Distance. Each box indicates the median (horizontal line inside the box), Q1 (first quartile = lower hinge), Q3 (third quartile = upper hinge), and mean (x). The upper whisker extends from the hinge to the largest value no further than 1.5 * IQR from the hinge (IQR = inter-quartile range). The lower whisker extends from the hinge to the smallest value at most 1.5 * IQR of the hinge. Data beyond the end of the whiskers are plotted individually and defined as outliers, indicated as °. Statistically significant comparisons (p < 0.05) were represented by * over a link bar.
Spirometric and 6-MWT parameters along the post-COVID-19 follow up period.
Moreover, among the patients who maintained FVC below the PV, three suffered pulmonary thromboembolism associated with parenchymal pulmonary disease during acute COVID-19, and all presented desaturation. All these patients presented > 50% pulmonary parenchymal involvement in CT, and none required orotracheal intubation.
DiscussionOur data showed that 6-months after the acute phase, symptoms, decreased pulmonary function, and low performance in the 6-MWT still persisted in approximately 28%, 16%, and 25% patients after severe COVID-19 disease, respectively. Since we excluded patients with suggestive previous pulmonary or cardiac disease, our data represent strong evidence of possible sequelae of COVID-19
Previous reports indicated persistent symptoms after COVID-19 ranging from 40%‒76%.6,17–19 However, part of these findings may be due to previous impairment of functional capacity. Other studies also have shown progressive improvement in functional parameters at 30, 90, 100, and 180 days after COVID-197,19–24 but, approximately one-third of the patients still had at least one symptom, and one-third presented with reduced FVC or low exercise capacity 6-months after hospital discharge, showing the possibility of a persistent condition in survivors severe COVID-19.
In the subset of five patients with persistent low pulmonary function at D180 and presenting at least one symptom, four of them also showed desaturation on the 6-MWT, and in three the WD was < 400 m, suggesting that the persistence of reduced pulmonary function and low exercise capacity may be associated with chronic disease. It is important to emphasize that 3 individuals in this subgroup also presented pulmonary thromboembolic event during acute phase, suggesting that vascular inflammation may be an important factor for the development of the chronic impairment of pulmonary function.
In our study dyspnea was the most frequent complaint and remained the most prevalent symptom 180 days after COVID-19, which agrees with other studies.6,8,22,25 The causes of dyspnea may be subjective, such as psychiatric disorders or muscular weakness, neuropathy, and general fatigue.6,24
Regarding respiratory tests, the isolated evaluation of FVC showed a progressive increase in pulmonary volume at D30, D90, and D180 (mean 496 ± 15 mL), mainly in the range of D30 to D90, in accordance with the literature data.22,23,26 This increase, although significant, was not enough to achieve an FVC ≥ 80% of PV in 5 (17%) patients in the D180 assessment. Therefore, despite the improvement, these data suggest that pulmonary changes tend to regress slowly and are present for at least 6-months after the acute phase. The restriction pattern may be explained by severe obesity, but this was not applicable in our study, as patients with severe obesity were excluded.
Moreover, the impairment of exercise capacity also improved in the first 3-months after discharge. A WD of < 75% PV was observed in a significant number of patients and was not related to low FVC, suggesting the existence of other mechanisms in pulmonary lesions, including impairment of pulmonary microvasculature. The reduced WD and desaturation in the 6-MWT may be related to low DLCO;22,27–29 the 6-MWT is a good and simple follow-up test for these patients because it indicates possible limitations to performing daily activities with social and economic impacts, as well as the necessity for improved health services to accommodate new patients with specific needs.
Pulmonary sequelae persist as a challenge due to the high and still increasing number of severe COVID-19 survivors, besides doubts about how to proceed with these patients and how to avoid complications.
Few follow-up studies with a considerable number of survivors of severe form of COVID-19 have evaluated the recovery of pulmonary function and functional capacity after hospital discharge. Most investigators had difficulty defining pulmonary sequelae due to the high prevalence of previous comorbidities associated with severe COVID-19.30 These comorbidities may be confounders that interfere with the interpretation of respiratory symptoms, pulmonary function variables or reduced exercise capacity. To avoid this problem, we excluded from our study all patients reporting previous comorbidities, such as heart and pulmonary diseases, and those with extrapulmonary complications from COVID-19. Thus, it is likely that symptoms that remained long after COVID-19 recovery were due to sequelae of the disease and not to previous comorbidities.
Our study also has limitations and data should be viewed cautiously. The first, was the small number of patients who completed the follow up. Another limitation was that the diffusing capacity of the lungs for carbon monoxide (DLCO) and Total Lung Capacity (TLC) were not measured. Considering that reductions in DLCO and TLC are frequent abnormalities in COVID-19, followed by reduced FVC,23 respiratory limitations may be more common than that reported in our study. Low DLCO is related to anatomical damage, resulting in interstitial or pulmonary vascular abnormalities.31 This possibility is corroborated by persistent dyspnea and poor exercise capacity in some patients. Another limitation was that 13 patients dropped out of the study. A possible explanation for this is the progressive improvement over time, considering that the majority dropped out after the third month of follow-up and only two individuals dropped out in the first 3-months.
ConclusionsMost patients with severe COVID-19 showed progressive improvement in both respiratory function and symptoms along the six months after acute recovery of severe COVID-19 especially in the first 3-months after hospitalization. However, the persistence of symptoms in addition to changes in both pulmonary function and decreased exercise capacity suggest the development of long COVID-19 in some patients undergoing or not undergoing intubation and mechanical ventilation. Further studies and follow-ups enrolling a larger number of patients and for a longer period of time are needed to establish the persistent sequelae and associated risk factors after COVID-19.
Institution where the article was developed/written: Federal University of Espírito Santo.