COVID-19-Associated Pulmonary Aspergillosis (CAPA) is a relatively common complication in patients with severe forms of the disease caused by the SARS-CoV-2 virus. Diagnosing and confirming CAPA is challenging. In this study, Aspergillus spp. isolation in respiratory specimens from patients with COVID-19 was evaluated for identifying cases of CAPA. In 2020‒2021, 17 Aspergillus spp. were isolated from 15 COVID-19 patients admitted to a university hospital in Brazil. Patient records were retrospectively reviewed to obtain clinical-epidemiological data and other markers of Aspergillus spp. infection and then compared with the ECMM/ISHAM criteria for defining CAPA. Probable CAPA was defined in 5/10 patients, who had Aspergillus spp. isolated from Bronchoalveolar Lavage (BAL) or a positive galactomannan blood test. Additionally, anti-Aspergillus antibodies were detected in two of these patients, during active or follow-up phases of CAPA. In another seven patients with Aspergillus spp. isolated from tracheobronchial aspirate or sputum, CAPA was presumed, mainly due to deterioration of clinical conditions and new lung imaging suggestive of fungal infection. Antifungal agents to control CAPA, particularly voriconazole, were used in 9/15 cases. In cases of probable CAPA and remaining patients, clinical conditions and comorbidities were similar, with lethality being high, at 60% and 71%, respectively. The number of CAPA cases defined by scientific criteria was lower than that assumed in the clinical context. This was largely due to the lack of BAL collection for fungal culture and the non-intensive use of other markers of invasive aspergillosis. The isolation of Aspergillus spp. in different respiratory specimens should alert clinicians to the diagnosis of CAPA.
SARS-CoV-2 infection promotes opportunistic infections by damaging lung parenchyma and impairing immune responses, particularly in patients with diabetes mellitus, chronic visceral diseases, and other comorbidities.1 Severe COVID-19 cases are admitted to Intensive Care Units (ICUs) and undergo invasive procedures and immunosuppressive therapy, which are additional factors for nosocomial infections by various types of microorganisms.2,3
Aspergillus spp. has been recognized as the main cause of fungal lung infection in patients with COVID-19,4 which has been termed COVID-19-Associated Pulmonary Aspergillosis (CAPA). CAPA pathogenesis is not fully understood, but there is evidence that it is more focal and related to airways, unlike those more angioinvasive opportunistic aspergillosis observed in neutropenic patients.5 Clinically presuming and confirming CAPA may be a difficult task. In general, symptoms, signs, and radiographic changes are common to other lung diseases, including those resulting from SARS-CoV-2 infection. Aspergillus spp. isolation from respiratory specimens can be alert for CAPA, but it may only represent patient colonization or contamination.6,7
Consensus and guidelines by medical and scientific societies have proposed diagnosis of pulmonary aspergillosis at progressive levels of evidence in patients with cancer or immunosuppressed,8 admitted to ICUs,9 and infected with influenza10 or COVID-19.11 Major evidence of invasive aspergillosis includes observation or isolation of Aspergillus spp., or detection of its antigenic or molecular components in clinical specimens less prone to colonization, such as lung and bronchial biopsies, Bronchoalveolar Lavage (BAL), and blood. A consensus developed by experts from the European Confederation of Medical Mycology and the International Society of Human and Animal Mycology (ECMM/ISHAM) for diagnosis of CAPA establishes, as a preliminary condition, that patients have clinical manifestations and radiographic findings compatible with invasive pulmonary aspergillosis, in addition to exclusion of other infectious agents. Depending on the type of laboratory evidence, the ECMM/ISHAM consensus proposes three levels of diagnostic evidence, which are summarized as follows: a) Proven CAPA – observation, isolation, or positive PCR for Aspergillus spp. in lung or bronchial tissue; b) Probable CAPA – microscopic observation or isolation of Aspergillus spp. in BAL and/or detection of significant levels of galactomannan or positive PCR for Aspergillus spp. in BAL and/or blood; c) Possible CAPA – microscopic observation or isolation of Aspergillus spp. in non-BAL bronchial lavage or detection of elevated or repeated levels of antigens or nucleic acid of this fungus in non-BAL bronchial lavage. The consensus also defines a tracheobronchitis form of CAPA as a bronchial mucosal injury suggestive of fungal infection, along with mycological, antigenic, or molecular evidence of Aspergillus spp. in bronchial biopsy or BAL.11
In this study, the isolation of Aspergillus spp. in respiratory specimens from patients with severe COVID-19 was the starting point to identify and classify CAPA cases according to the ECMM/ISHAM criteria and to highlight challenges in confirming and ruling out the diagnosis of invasive aspergillosis in these patients.
Materials and methodsThis retrospective study was conducted at the Hospital of Clinicas of Ribeirão Preto Medical School, University of São Paulo, located in São Paulo State, Brazil. The hospital is a tertiary-level medical care center and a referral for COVID-19 patients. Between June 2020 and December 2021, 135 samples of Aspergillus spp. were isolated in the hospital's Mycology Laboratory, 18 of which were obtained by culturing respiratory specimens from 16 hospitalized COVID-19 patients. One Aspergillus spp. isolate became non-viable, leaving 17 isolates from 15 patients with COVID-19 whose clinical data and laboratory diagnosis of presumed CAPA was compiled from medical records.
The 15 patients were admitted due to respiratory failure. All had SARS-CoV-2 infection detected by real-time PCR. The standardized therapeutic regimen for patients with COVID-19 consisted of azithromycin, ceftriaxone, corticosteroids (dexamethasone, methylprednisolone, or hydrocortisone), and anticoagulants. Additional medications were introduced when necessary to treat complications. Reasons for requesting fungal culture in airway specimens included persistent fever despite the use of antibacterial drugs and no respiratory recovery or worsening, concurrent with lung imaging changes, particularly if suggestive of fungal infection. In three patients, Aspergillus spp. was isolated from tracheal aspirate collected due to suspected bacterial pneumonia.
Sabouraud agar culture medium was used for fungal isolation in Tracheobronchial Aspirate (TA) (n = 12), BAL (n = 3), and sputum (n = 2). Identification of Aspergillus spp. was initially performed by observing the macro and micro-morphological characteristics of each Aspergillus spp. isolate. The Aspergillus spp. isolates were identified using the MALDI-TOF MS method by extracting proteins via the solid Sabouraud Dextrose Agar Oxoid™ culture medium method.12 The Bruker IVD MALDI BIOTYPER™ MICROFLEX™ LT/SH platform (Bruker Daltonick GmbH & Co. KG, Bremen, Germany) was used at the Hospital of Clinics of Ribeirão Preto Medical School and the Adolfo Lutz Institute's Culture Collections Center, São Paulo, Brazil. The spectra of each Aspergillus spp. were evaluated in the Mass Spectrometry Identification (MSI 2.0) database available at http://msi.happy-dev.fr, using the identification criteria recommended by the software.
Galactomannan was measured in the blood (n = 7) or BAL (n = 2) of 8 patients using an Enzyme-Linked Immunosorbent Assay (ELISA) on a microplate. The PLATELIA™ Aspergillus Ag kit from Bio-Rad Laboratories was used. The measured index in optical density was considered positive when ≥ 0.5 in blood samples and ≥ 1.0 in BAL samples.
The Counterimmunoelectrophoresis (CIE) test for anti-Aspergillus spp. antibodies was performed in 6/15 patients. For this test, a pool of antigens from three strains of Aspergillus spp. was used, which were obtained by culture in McVeigh Morton liquid medium for 60 days, followed by filtration, dialysis, and concentration of the culture broth. Electrophoresis of serum against the antigen pool was performed with an electric current of 15 mA for 60 min.13
Cases of probable CAPA were classified according to the criteria established by the ECMM/ISHAM consensus.11 Patients who did not meet the criteria were grouped as clinically suspected or non-suspected CAPA.
The study was approved by the Ethics Committee of the Hospital of Clinics of Ribeirão Preto Medical School, under protocol number 54893722.1.0000.5440.
ResultsTable 1 shows the main laboratory findings related to aspergillosis diagnosis and clinical and radiological data from 15 COVID-19 cases. All patients, except for two, were over 50 years old. Aspergillus spp. was isolated in 17 cases, identified as A. flavus/oryzae (n = 6), A. fumigatus (n = 7), A. tamarii (n = 2), A. niger (n = 1), and A. terreus (n = 1). In case 5, A. fumigatus was isolated from both BAL and sputum.
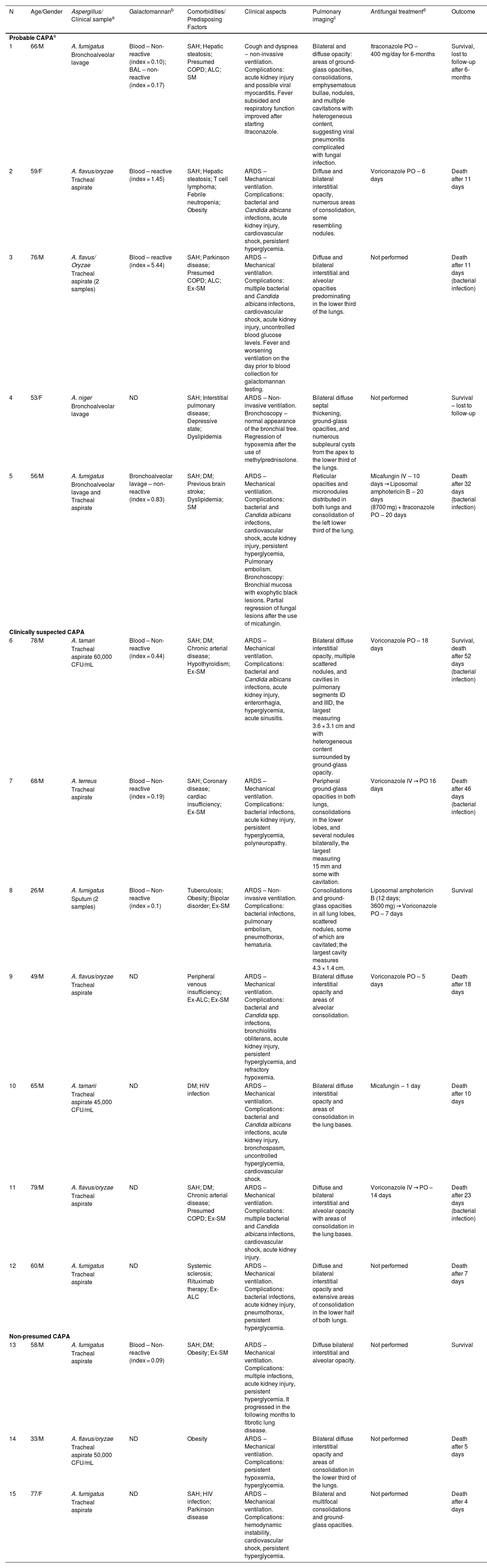
Laboratory diagnosis, clinical and radiographic aspects, antifungal treatment, and outcomes of patients with probable or presumed COVID-19-Associated Pulmonary Aspergillosis (CAPA).
| N | Age/Gender | Aspergillus/ Clinical samplea | Galactomannanb | Comorbidities/ Predisposing Factors | Clinical aspects | Pulmonary imagingc | Antifungal treatmentd | Outcome |
|---|---|---|---|---|---|---|---|---|
| Probable CAPAe | ||||||||
| 1 | 66/M | A. fumigatus | Blood – Non-reactive (index = 0.10); BAL – non-reactive (index = 0.17) | SAH; Hepatic steatosis; Presumed COPD; ALC; SM | Cough and dyspnea ‒ non-invasive ventilation. Complications: acute kidney injury and possible viral myocarditis. Fever subsided and respiratory function improved after starting itraconazole. | Bilateral and diffuse opacity: areas of ground-glass opacities, consolidations, emphysematous bullae, nodules, and multiple cavitations with heterogeneous content, suggesting viral pneumonitis complicated with fungal infection. | Itraconazole PO ‒ 400 mg/day for 6-months | Survival, lost to follow-up after 6-months |
| Bronchoalveolar lavage | ||||||||
| 2 | 59/F | A. flavus/oryzae | Blood – reactive (index = 1.45) | SAH; Hepatic steatosis; T cell lymphoma; Febrile neutropenia; Obesity | ARDS ‒ Mechanical ventilation. Complications: bacterial and Candida albicans infections, acute kidney injury, cardiovascular shock, persistent hyperglycemia. | Diffuse and bilateral interstitial opacity, numerous areas of consolidation, some resembling nodules. | Voriconazole PO ‒ 6 days | Death after 11 days |
| Tracheal aspirate | ||||||||
| 3 | 76/M | A. flavus/ Oryzae | Blood – reactive (index = 5.44) | SAH; Parkinson disease; Presumed COPD; ALC; Ex-SM | ARDS ‒ Mechanical ventilation. Complications: multiple bacterial and Candida albicans infections, cardiovascular shock, acute kidney injury, uncontrolled blood glucose levels. Fever and worsening ventilation on the day prior to blood collection for galactomannan testing. | Diffuse and bilateral interstitial and alveolar opacities predominating in the lower third of the lungs. | Not performed | Death after 11 days (bacterial infection) |
| Tracheal aspirate (2 samples) | ||||||||
| 4 | 53/F | A. niger | ND | SAH; Interstitial pulmonary disease; Depressive state; Dyslipidemia | ARDS ‒ Non-invasive ventilation. Bronchoscopy ‒ normal appearance of the bronchial tree. Regression of hypoxemia after the use of methylprednisolone. | Bilateral diffuse septal thickening, ground-glass opacities, and numerous subpleural cysts from the apex to the lower third of the lungs. | Not performed | Survival – lost to follow-up |
| Bronchoalveolar lavage | ||||||||
| 5 | 56/M | A. fumigatus | Bronchoalveolar lavage – non-reactive (index = 0.83) | SAH; DM; Previous brain stroke; Dyslipidemia; SM | ARDS – Mechanical ventilation. Complications: bacterial and Candida albicans infections, cardiovascular shock, acute kidney injury, persistent hyperglycemia, Pulmonary embolism. Bronchoscopy: Bronchial mucosa with exophytic black lesions. Partial regression of fungal lesions after the use of micafungin. | Reticular opacities and micronodules distributed in both lungs and consolidation of the left lower third of the lung. | Micafungin IV ‒ 10 days → Liposomal amphotericin B ‒ 20 days (8700 mg) + Itraconazole PO ‒ 20 days | Death after 32 days (bacterial infection) |
| Bronchoalveolar lavage and Tracheal aspirate | ||||||||
| Clinically suspected CAPA | ||||||||
| 6 | 78/M | A. tamari | Blood – Non-reactive (index = 0.44) | SAH; DM; Chronic arterial disease; Hypothyroidism; Ex-SM | ARDS ‒ Mechanical ventilation. Complications: bacterial and Candida albicans infections, acute kidney injury, enterorrhagia, hyperglycemia, acute sinusitis. | Bilateral diffuse interstitial opacity, multiple scattered nodules, and cavities in pulmonary segments ID and IIID, the largest measuring 3.6 × 3.1 cm and with heterogeneous content surrounded by ground-glass opacity. | Voriconazole PO – 18 days | Survival, death after 52 days (bacterial infection) |
| Tracheal aspirate 60,000 CFU/mL | ||||||||
| 7 | 68/M | A. terreus | Blood – Non-reactive (index = 0.19) | SAH; Coronary disease; cardiac insufficiency; Ex-SM | ARDS – Mechanical ventilation. Complications: bacterial infections, acute kidney injury, persistent hyperglycemia, polyneuropathy. | Peripheral ground-glass opacities in both lungs, consolidations in the lower lobes, and several nodules bilaterally, the largest measuring 15 mm and some with cavitation. | Voriconazole IV → PO 16 days | Death after 46 days (bacterial infection) |
| Tracheal aspirate | ||||||||
| 8 | 26/M | A. fumigatus | Blood – Non-reactive (index = 0.1) | Tuberculosis; Obesity; Bipolar disorder; Ex-SM | ARDS – Non-invasive ventilation. Complications: bacterial infections, pulmonary embolism, pneumothorax, hematuria. | Consolidations and ground-glass opacities in all lung lobes, scattered nodules, some of which are cavitated; the largest cavity measures 4.3 × 1.4 cm. | Liposomal amphotericin B (12 days; 3600 mg) → Voriconazole PO – 7 days | Survival |
| Sputum (2 samples) | ||||||||
| 9 | 49/M | A. flavus/oryzae | ND | Peripheral venous insufficiency; Ex-ALC; Ex-SM | ARDS – Mechanical ventilation. Complications: bacterial and Candida spp. infections, bronchiolitis obliterans, acute kidney injury, persistent hyperglycemia, and refractory hypoxemia. | Bilateral diffuse interstitial opacity and areas of alveolar consolidation. | Voriconazole PO ‒ 5 days | Death after 18 days |
| Tracheal aspirate | ||||||||
| 10 | 65/M | A. tamarii | ND | DM; HIV infection | ARDS – Mechanical ventilation. Complications: bacterial and Candida albicans infections, acute kidney injury, bronchospasm, uncontrolled hyperglycemia, cardiovascular shock. | Bilateral diffuse interstitial opacity and areas of consolidation in the lung bases. | Micafungin ‒ 1 day | Death after 10 days |
| Tracheal aspirate 45,000 CFU/mL | ||||||||
| 11 | 79/M | A. flavus/oryzae | ND | SAH; DM; Chronic arterial disease; Presumed COPD; Ex-SM | ARDS – Mechanical ventilation. Complications: multiple bacterial and Candida albicans infections, cardiovascular shock, acute kidney injury. | Diffuse and bilateral interstitial and alveolar opacity with areas of consolidation in the lung bases. | Voriconazole IV → PO – 14 days | Death after 23 days (bacterial infection) |
| Tracheal aspirate | ||||||||
| 12 | 60/M | A. fumigatus | ND | Systemic sclerosis; Rituximab therapy; Ex-ALC | ARDS – Mechanical ventilation. Complications: bacterial infections, acute kidney injury, pneumothorax, persistent hyperglycemia. | Diffuse and bilateral interstitial opacity and extensive areas of consolidation in the lower half of both lungs. | Not performed | Death after 7 days |
| Tracheal aspirate | ||||||||
| Non-presumed CAPA | ||||||||
| 13 | 58/M | A. fumigatus | Blood – Non-reactive (index = 0.09) | SAH; DM; Obesity; Ex-SM | ARDS ‒ Mechanical ventilation. Complications: multiple infections, acute kidney injury, persistent hyperglycemia. It progressed in the following months to fibrotic lung disease. | Diffuse bilateral interstitial and alveolar opacity. | Not performed | Survival |
| Tracheal aspirate | ||||||||
| 14 | 33/M | A. flavus/oryzae | ND | Obesity | ARDS ‒ Mechanical ventilation. Complications: persistent hypoxemia, hyperglycemia. | Bilateral diffuse interstitial opacity and areas of consolidation in the lower third of the lungs. | Not performed | Death after 5 days |
| Tracheal aspirate 50,000 CFU/mL | ||||||||
| 15 | 77/F | A. fumigatus | ND | SAH; HIV infection; Parkinson disease | ARDS ‒ Mechanical ventilation. Complications: hemodynamic instability, cardiovascular shock, persistent hyperglycemia. | Bilateral and multifocal consolidations and ground-glass opacities. | Not performed | Death after 4 days |
| Tracheal aspirate | ||||||||
ARDS, Acute Respiratory Distress Syndrome; SAH, Systemic Arterial Hypertension; DM, Diabetes Mellitus 2; COPD, Chronic Obstructive Pulmonary Disease; ALC, Chronic Alcoholism; SM, Chronic Smoking.
Galactomannan antigen blood test was positive in 2 out of 7 patients and negative in 2 out of 2 patients in the BAL testing. Serum anti-Aspergillus antibodies were detected in 1 out of 6 patients (case 5 ‒ titer 1:16). In case 1, these antibodies were absent during the episode of COVID-19, but the test became positive (titer 1:8) four months later.
According to the ECMM/ISHAM criteria, 5 cases were classified as probable CAPA (cases 1 to 5). Case 5 presented with the tracheobronchitis form of CAPA, based on the presence of exophytic fungal lesions in the bronchi. CAPA was clinically presumed but not confirmed in 7 patients, and there was no clinical suspicion of CAPA in the other 3 cases. The period between hospital admission and isolation of Aspergillus spp. was 2 to 25 (median = 11) days for patients with probable CAPA and 7 to 44 (median = 12) days for the other cases.
All 15 patients were admitted due to respiratory failure and required supplemental oxygen, with 13 out of 15 receiving mechanical ventilation and intensive care. Most patients had one or more opportunistic bacterial infections, particularly pneumonia, and sepsis, and 7 had urinary candidiasis before the isolation of Aspergillus spp. All 15 patients had comorbidities, with diabetes mellitus (n = 5), presumed chronic obstructive pulmonary disease ‒ COPD (n = 3), interstitial lung disease (n = 1), immunosuppression (n = 2), and frequent reports of current or past smoking and/or alcoholism being the most common conditions predisposing to aspergillosis. Corticosteroids were used in the treatment of COVID-19 in all patients, with 12 receiving them for more than a week. Persistent and difficult-to-control hyperglycemia and acute kidney injury worsened the clinical conditions of many patients.
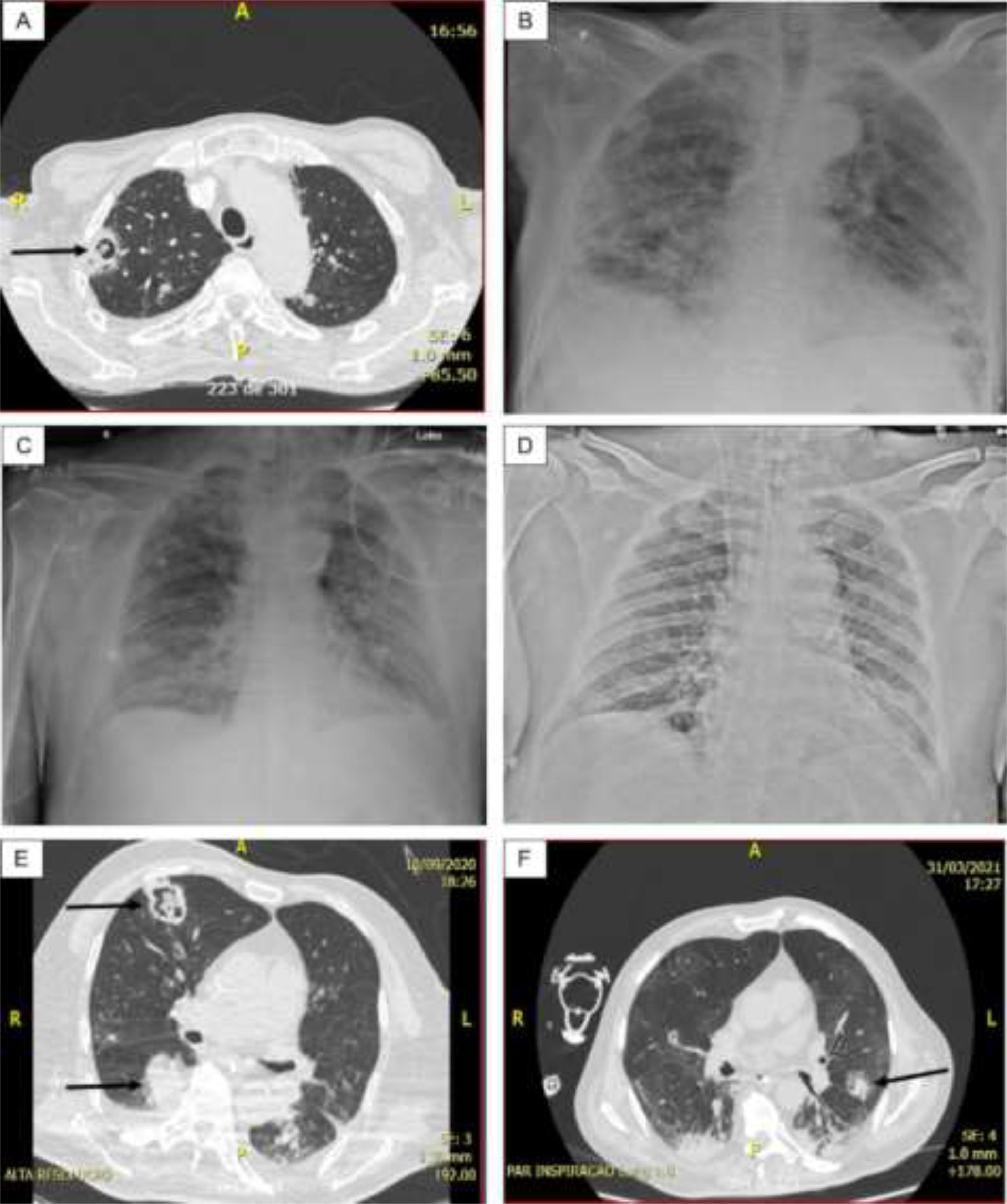
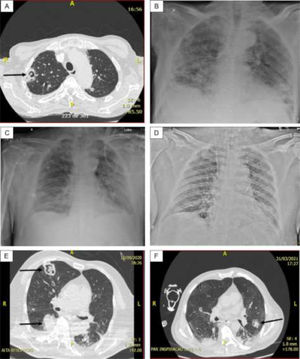
The most common radiographic finding in the 15 patients, generally attributed to SARS-CoV-2 infection, was bilateral interstitial pulmonary infiltrate associated with consolidations and areas of ground-glass opacity. Cavitations (n = 4) and/or pulmonary nodules with a diameter ≥ 1.0 cm (n = 5) were observed in cases 1, 2 (probable CAPA), 6, 7, and 8 (presumed CAPA) and considered suggestive of fungal infection (associated with pulmonary tuberculosis in case 8) (Fig. 1).
(A) Case 1 – Thorax CT: Cavitary lesion (arrow) and numerous nodules in both lungs; (B) Case 1 – Thorax radiography: Diffuse consolidations, ground glass areas and nodules in both lungs. (C) Case 3 – Thorax radiography: Diffuse interstitial opacities and consolidations, some of that resembling nodules; (D) Case 5 – Thorax radiography: Bilateral and diffuse interstitial opacities containing micronodules and condensation in the lower lobe of left lung; (E) Case 6 – Thorax CT: Diffuse interstitial opacities, cavitary lesion with inner amorphous mass (large arrow), macronodule (thin arrow), and condensation in the right lung; (F) Case 7 – Thorax CT: ground glass opacities, macronodule (arrow) and peripheral consolidations in both lungs.
Antifungal therapy was used or at least initiated in 3 out of 5 cases of probable CAPA and in 6 out of 7 cases of clinically presumed CAPA, mainly with voriconazole, but also with liposomal amphotericin B, micafungin, and itraconazole. In the 6 out of 12 patients who survived the episode of probable or presumed CAPA, the course of antifungal therapy lasted between 14 days and 6 months, with a median of 18.5 days. Two patients with probable CAPA did not receive antifungal therapy, either because of clinical stabilization with the use of corticosteroids (case 4) or death due to concomitant bacterial infection and CAPA (case 3).
At the end of hospitalization, death occurred in 3 out of 5 (60%) cases of probable CAPA and 5 out of 7 (71%) cases of clinically presumed CAPA. The cause of death was not only Aspergillus spp. infection, but also mainly bacterial infections and other complications of COVID-19.
DiscussionThis study found that isolating Aspergillus spp. from COVID-19 patients allowed to retrospectively define five probable cases of CAPA using the ECMM/ISHAM criteria.11 However, the decision to use antifungal therapy did not always match the level of proof of aspergillosis. Along with isolation of Aspergillus spp., using antifungals for presumptive CAPA was justified by the patient's clinical conditions of non-recovery or worsening respiratory symptoms, and the onset of changes suggestive of fungal infection in new lung images. Some cases of suspected aspergillosis (6, 7, 8, and others) could have been classified as probable or proven CAPA if accepted procedures and tests were used.14,15 Testing BAL is essential for diagnosing CAPA, but finding Aspergillus spp. in BAL samples does not necessarily prove infection.16 On the other hand, case 4, which was classified as probable CAPA, did not have clinically recognized aspergillosis. Clinical case studies and autopsies have shown some failures in the application of different consensus criteria in defining and excluding CAPA cases.5,17
Applying ECMM/ISHAM criteria in the clinical context of COVID-19 is somewhat challenging. BAL and lung tissue or bronchial biopsy require bronchoscopy, which is often avoided due to the patient's critical condition and the risk of SARS-CoV-2 transmission, reducing the chance of confirming CAPA. Alternatively, probable CAPA can also be defined by detecting significant levels of galactomannan in the blood. Although this test is easily obtained, its sensitivity in non-neutropenic COVID-19 patients may be less than 50%.7 Galactomannan levels in the blood were positive in two patients in this study, allowing for their inclusion in the probable CAPA group, but it was negative in another patient in this group. Isolation of Aspergillus spp. in different respiratory specimens from COVID-19 patients is a valuable tool in presuming CAPA and, along with clinical and radiological data, can support the use of antifungal agents.18 However, isolating Aspergillus spp. in non-BAL specimens is not enough to prove CAPA according to the ECMM/ISHAM criteria as it does not necessarily indicate Aspergillus infection. The easier collection of non-BAL tracheal aspirate and bronchial lavage samples has led to studies on the predictive value of detecting Aspergillus spp. in these specimens.19,20 The diagnostic usefulness of Aspergillus spp. colony counts in the tracheal aspirate of some patients in this study is not known.
Chances of finding anti-Aspergillus spp. antibodies in CAPA cases are small, as the fungal infection is recent, and the patient's immune response is decreased in intensity. Therefore, this test is not included in the defining criteria for acute invasive aspergillosis. However, due to its non-invasiveness and availability, a serological test was performed on some patients, with a positive result obtained in 1/3 of probable CAPA cases. In one patient with a negative test, anti-Aspergillus antibodies appeared during follow-up. The presence of anti-Aspergillus precipitins was previously demonstrated in cases of CAPA,21 and the search for these antibodies may be more useful for diagnosing aspergillosis in COVID-19 patients in better clinical condition or with a subacute form of the fungal infection.22
The identification of Aspergillus species and patients’ clinical characteristics did not allow us to distinguish probable CAPA cases from others. A. flavus/oryzae and A. fumigatus were the most identified species both in probable CAPA and in other patients. A. fumigatus has been the predominant species found in CAPA cases, followed by A. flavus and other less prevalent species, but there is regional variation.23
Many patients with probable or presumed CAPA had critical and unstable clinical conditions, presenting with Acute Respiratory Distress Syndrome (ARDS), and requiring mechanical ventilation, in addition to numerous pulmonary and systemic complications. Interestingly, the only two patients in this study who were not in ICUs were diagnosed with CAPA due to their better clinical condition, which allowed bronchoscopy and BAL collection. The estimated median times for diagnosis of probable or presumed CAPA was 11 and 12 days, respectively. Generally, CAPA appears within two weeks after admission to the ICU, but late-onset aspergillosis can occur in patients with persistent pulmonary complications and/or prolonged mechanical ventilation.23 Comorbidities of patients with probable or possible CAPA have been observed in other studies, including diabetes mellitus, hypertension, obesity, and other chronic organic diseases.24 All evaluated patients had known predisposing factors for aspergillosis, including current or past smoking and alcoholism, diabetes mellitus, use of corticosteroids, and/or immunosuppression.2 Among the five cases of probable CAPA, two had presumed Chronic Obstructive Pulmonary Disease (COPD), one patient was investigating interstitial lung disease, and one patient had COVID-19 and CAPA while presenting with febrile neutropenia after chemotherapy for T-cell lymphoma. Previous bronchopulmonary disease has also been associated with CAPA.2,25
In some patients with CAPA, changes in lung imaging exams are non-specific and overlap with radiographic findings of COVID-19.26,27 Nodules and cavitations appearing during COVID-19 may suggest aspergillosis and lead to diagnosis and treatment of presumed CAPA, but they can also be caused by Mucorales and other fungi.28Aspergillus spp. has been commonly associated with macronodules and/or pulmonary cavitation in COVID-19 patients.29 Bronchial wall thickening and pulmonary condensation in initial imaging exams of COVID-19 patients have also been associated with CAPA.30 Another major differential diagnosis for the presence of nodular and cavitary lesions in COVID-19 patients with a prolonged course is tuberculosis.31 The co-occurrence of these infections seems to be associated with a worse prognosis, as in the case of CAPA.
Voriconazole has been the preferred antifungal for treating CAPA, as in this series of probable or presumed CAPA cases.23 Other azolic drugs, liposomal amphotericin B and micafungin are alternatives for antifungal therapy in CAPA.32 A few weeks of antifungal drug use were enough to control probable or presumed CAPA. Experts recommend a therapeutic course of 6 to 12 weeks,11 but one patient in this study used itraconazole for a longer period, which was necessary to reduce lung changes. The mortality rate in probable CAPA cases (60%) is high, but it is within the range observed in other case series.23,32
This study has limitations due to its retrospective nature, lack of standardization in clinical investigation of invasive aspergillosis in COVID-19 patients, and the small number of patients included.
There are discrepancies between clinical suspicion and scientific confirmation of acute invasive aspergillosis in COVID-19 patients. In the presence of clinical-radiological changes suggestive of CAPA, the medical staff tends to initiate antifungal therapy even without meeting a minimum laboratory criterion defined by specialists. Moreover, the isolation of Aspergillus spp. in respiratory specimens from COVID-19 patients can be the initial finding to presume and confirm CAPA.
Authors’ contributionsT. A. Cocio performed isolation, morphologic identification and maintenance of fungi in culture of Aspergillus spp., all methodological and analysis steps of MALDI-TOF identification; assisted in the analysis of the epidemiological data of the CAPA cases evaluated in this study; idealized the concept of the study and revised the manuscript text; L.M.P. Siqueira performed extraction of proteins and MALDI-TOF analysis of spectras of each Aspergillus spp. clinical isolate obtain in this study and revised the manuscript text; K.C.T. Riciluca performed the MALDI-TOF obtain of spectras and analysis in the M.S.I data bank and revised the manuscript text; V.M.F. Gimenes and T.S. de Andrade revised all the results of MALDI-TOF identification; G. Benard revised all epidemiological data of CAPA cases and revised the manuscript text; R. Martinez analyzed all the epidemiological data of each patient with CAPA evaluated in this study, analyzed all serological datas, idealized the study concept and wrote the manuscript; V. R. Bollela coordinate, guide and plan the entire methodology execution, idealized the main concept of the study, obtained financial resources for the execution of the work and revised the manuscript text.
FundingThe study was supported by the Foundation for the Support of Teaching, Research, and Medical Assistance of the Hospital das Clínicas of the Ribeirão Preto Medical School – FAEPA and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for granting the post-doctoral scholarships (Grant numbers: 150639/2022-8 (T.A.Cocio)).
We thank the biologists Cristiane Januário Sette, Daniela Maria Vieira Ferracioli Aguetoni from the Laboratory of Microbiology and Mycology at Hospital das Clínicas de Ribeirão Preto ‒ HCFMRP/USP, for their help in the morphological identification of Aspergillus spp. and Leula Maria de Almeida Pinheiro from the HCFMRP/USP serology laboratory collaborated in the execution of the serological tests.