Leishmaniasis is transmitted by sandfly which carries the intracellular protozoa in their midgut. Among visceral, cutaneous and mucocutaneous leishmaniasis, visceral type that is caused by Leishmania donovani is the most lethal one. Findings of leishmanial structure and species took place in 19th century and was initiated by Donovan. Leishmaniasis is still a major concern of health issues in many endemic countries in Asia, Africa, the Americas, and the Mediterranean region. Worldwide1.5-2 million new cases of cutaneous leishmaniasis and 500,000 cases of visceral leishmaniasis are reported each year. Leishmaniasis is endemic in nearly 90 countries worldwide and close to 12 million new cases of leishmaniasis are reported worldwide annually. Studies on antileishmanial drug development is of major concern as leishmaniasis are the second largest parasitic killer in the world and the available drugs are either toxic or costly. The major surface GP63 protease, also known as Zinc- metalloproteases present on the surface of leishmanial promastigotes, can be targeted for drug development. Protease inhibitors targeting such surface proteases show promising results. Different protease inhibitors have been isolated from marine actinobacteria against many infectious diseases. Metabolites produced by these actinobacteria may have greater importance for the discovery and development of new antileishmanial drugs. Hence, this review discusses the background, current situation, treatment, and protease inhibitors from marine actinobacteria for drug development against GP63 molecules.
Leishmaniasis is an intracellular protozoa-based parasite infection that is spread via a female sandfly bite. Leishmaniasis has a wide clinical spectrum, ranging from a self-curing, cutaneous ulcer to extensively widespread skin lesions, mucocutaneous disease, and even a lethal systemic infection disturbing the reticuloendothelial system.3 Due to more international ease, army-related travel, change of vector habitats, and other variables which promote vulnerability, such as infections like HIV and undernourishment, the global prevalence of leishmaniasis has amplified.15 Leishmania causes sickness in humans and in over 20 different species. The parasites have been found in broad parts of the planet, with the exclusion of Australia and Antarctica. Complications linked with subsequent infections may result in mortality. The kind of visceral leishmaniasis (VL) produced by L. donovani is significantly more dangerous. It is caused by an infection of blood cells in the lymphoid organs, primarily the spleen, bone marrow and liver and is typically fatal if left untreated. Hepato-splenomegaly, anemia, fever, cachexia and leucopenia are all symptoms of VL. Leishmaniasis has a long-term course that might take months or even years to develop. The majorly of leishmania species that are found in Asia, India and middle east countries causes VL; Leishmania species located in South America and Central America cause cutaneous, visceral, and mucocutaneous disease. The very-small dimensions of the protozoa impounded within blood cells of bone marrow, reticuloendothelial structure, and skin makes diagnosing leishmaniasis challenging.2 In the more severe types of the condition, therapy has long been challenging, and the advent of medication resistance has made it even more challenging. There is no effective vaccination for leishmaniasis. Hence the present paper reviewed the background, current situation, and treatment of leishmaniasis along with the drug target site GP63 and protease inhibitors from marine actinobacteria for drug development against these GP63 molecules.
Leishmaniasis and 20th centuryLeishmaniasis is the world's second-leading parasite killer. Leishmaniasis ranks next only to malaria and second only to dengue fever, from 5.6 to 6 million, among the 16 groups of neglected tropical diseases (NTDs) studied from 2001 to 2015.39 Due to naturally occurring or disasters made by man such as drought, hunger, earthquake, floods and civil conflicts, the disease frequently reaches epidemic extents in places with little endemicity. Afflicted people with poor background were the primarily subsidizers to policymakers' and even scientists' lack of interest in leishmaniasis, resulting in a dearth of appropriate diagnostics and chemotherapeutic drugs to allow active supervision and regulation of the leishmanial infection. Leishmaniasis potential link to the spread of acquired immunodeficiency syndrome (AIDS) has drawn attention to the growing hazard of co-infection with HIV, notably in India, East Africa, and Brazil.15
In November 1900, while serving with the British Army in India, a pathologist William Boog Leishman noticed egg-shaped creatures in post-mortem smears obtained from the spleen of a warrior who died at a place called Dum-Dum in Calcutta. Following that, he discovered alike structures in a rat that had been infected experimentally. In 1903, William reported his findings, claiming that the egg-shaped creatures were deteriorated trypanosomes and that condition, which William dubbed "Dum-dum fever," was a kind of Trypanosoma infection. After some weeks, a physiology Professor at Madras medical college, Charles Donovan released a research paper describing that he had discovered identical structures in spleen samples collected in life and by autopsy from local people in India with enlarged spleens and high fever.19 Donovan directed a slide of this parasite to Felix Etienne in Paris, a biologist, and asked him to show the slides to his countryman Charles Louis, an expert of protozoans at that time.53 Later, it was suggested by Laveran to be a novel Piroplasma parasite.35 In the meantime, medical practitioner Ronald Ross, commissioned by the government of India to do research on Kala-Azar in 1898, issued a report in November of 1903 remarking on Leishman and Donovan's findings of oval entities in the spleen flesh of patients with persistent splenomegaly and raised body temperature.52 Ross came to the conclusion that, oval bodies were not deteriorated Trypanosomes, rather a unique protozoan, and that the clinical image of the cases was alike to Kala-Azar. His findings also disagreed with suggestion of Laveran's and proposed that these oval bodies fitted in a new genus called Leishmania donovani in a follow-up publication (Ross, 1904). The debate over the nature of Leishman's bodies raged on for next year, but by the close of 1904, the name Leishmania donovani had gained widespread acceptance.23 In 1908, Charles Henry initially described the species Leishmania infantum in spleen of children suffering from anemia in Tunisia.42 In the same year, he discovered the parasite in dogs in Tunisia. Dogs have been considered as significant carrier hosts for visceral leishmaniasis since then.42
Present situation worldwideIn many endemic nations, leishmaniasis is still a major health issue. Several factors have contributed to the rise in leishmaniasis cases seen around the world through the last 25 years. Leishmaniasis is spreading to non-endemic places due to two factors: climate change and globalization.59 For example, the number of cases of leishmaniasis among international travelers has risen in recent decades.37 Furthermore, international blood flow has caused infection of Leishmania in people who never went to endemic leishmanial zones.56 The issue happened here was that no blood-bank checks for antileishmanial antibodies in blood preservations. Evidence suggests that global warming will cause the spreading of sand flies to expand northwards, perhaps resulting in leishmaniasis transmission in non-endemic areas in the upcoming days.6 The sandfly's vulnerability to cold climes, preference to only draw blood from humans or animals, support the interior expansion of specific species of Leishmania and limit the geographic spreading of leishmaniasis.
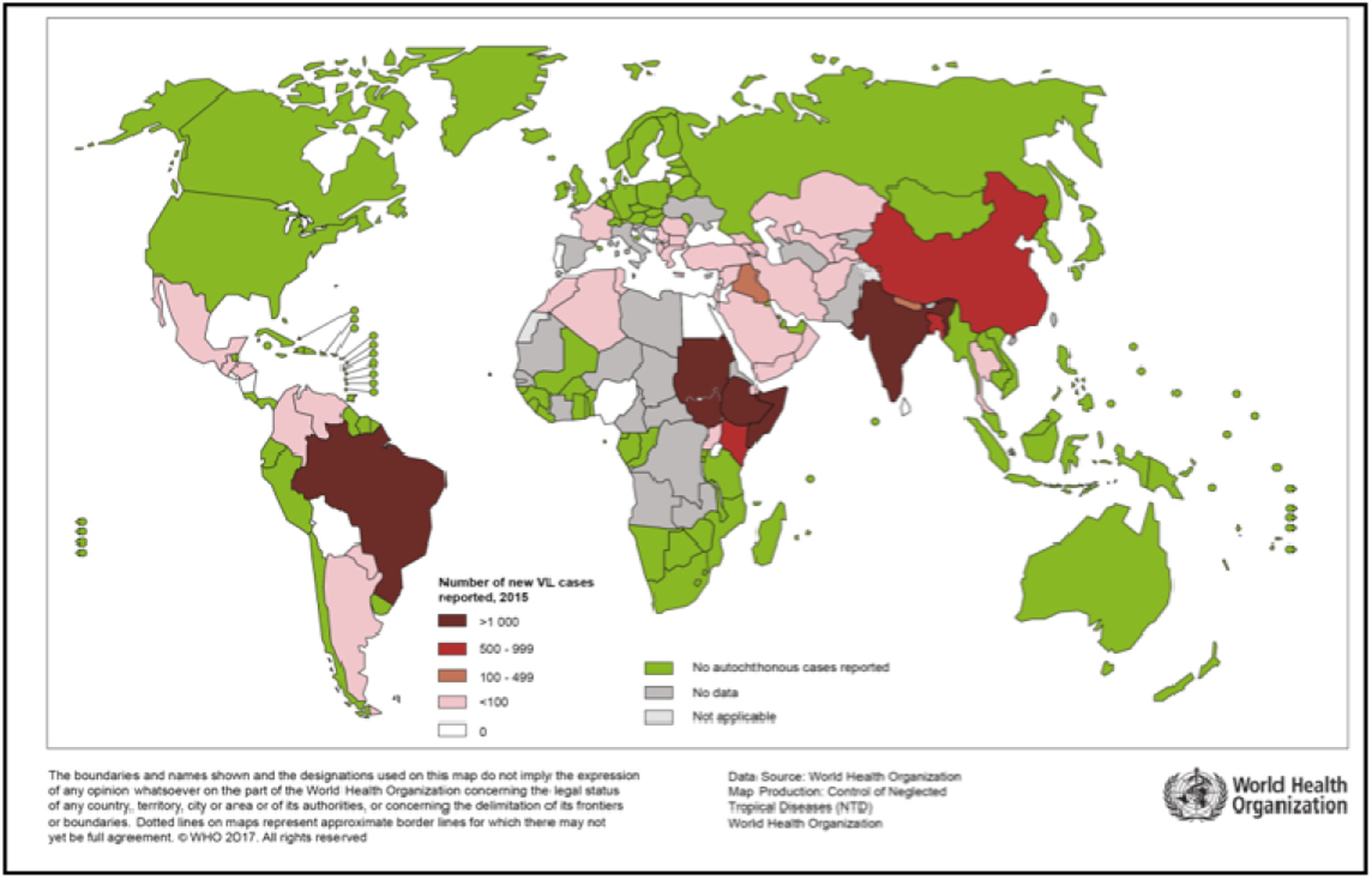
WHO reports prevalent leishmaniasis in 95 nations and five territories across five continents with a yearly incidence of 0.2-0.4 million cases of visceral illness.71 The Mediterranean Basin, the Americas, central Asian, and the Middle East, account for nearly all cases of cutaneous illness. More than two-thirds of leishmanial cases have been reported in Brazil, Iran and Syria, to name a few. Bangladesh, Ethiopia, Brazil, India, and Sudan account for almost 90% of new cases of visceral leishmaniasis.71 In approximately a three-year period in Colombia, the army fighting the Fuerzas Armadas of Colombia saw over 40,000 cases of leishmaniasis (Fig. 1). With 14,879 new cases stated in 2014, India has the highest burden of VL.75 Bolivia, Peru and Brazil account for about 90% of mucocutaneous leishmaniasis cases. Despite being surrounded by endemic leishmaniasis-endemic regions, Chile, Australia, Uruguay, the South Pacific, and Canada are not considered to have endemic leishmaniasis. In people living with AIDS, VL and CL is becoming recognized as possible opportunistic infections. More than 35 nations in southern Europe, Central and South America, the Mediterranean Basin, and India have documented HIV coinfection.70 The disease occurs as a result of severe immunosuppression. Because of the extensive use of antiretroviral medication, the frequency of coinfection has reduced in developed countries.
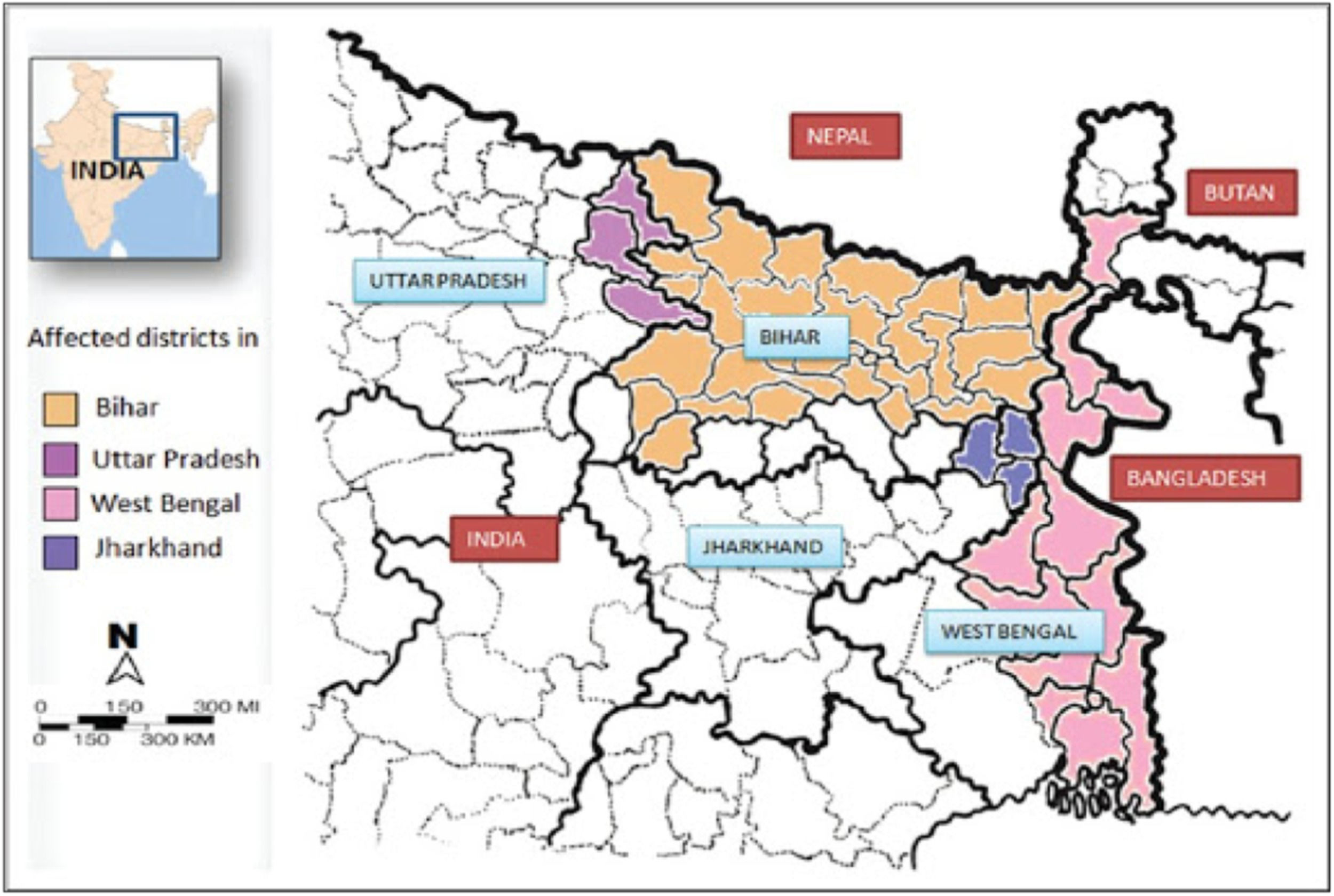
Bihar, India's largest state, accounts for roughly 90% of the cases. L. donovani has been continuously transmitted in Bihar for more than three decades, with more than 300,000 cases informed in the state from 1977.49 As demonstrated by a research undertaken in 2004 in Bihar, which confirmed a VL occurrence rate of 3.5 per 1000, 8-fold higher than the authorized figure, the officially declared cases represent a major underestimation of the genuine event cases.57 Several authors have highlighted the limitations in our knowledge of VL study and distribution in the Indian subcontinent, where risk factors for VL are still unknown.9 VL appears to have concentrated spreading in Bihar, occurring in tiny clusters with low socio-environmental conditions and limited access to the health system (Fig. 2).10 PKDL has implicated as a feature in the determination of L. donovani spread India1; though, the information on asymptomatically infected L. donovani infected persons in India and their possible part in the VL cycle is limited. In India, no such research has been conducted to determine the ratio of asymptomatic infections to clinical cases. All of these characteristics are critical for better understanding L. donovani transmission dynamics and developing more operative control programmes, which are presently based on unreceptive case exposure and treatment of VL patients, as well as control of vectors.
With hundreds of thousands of cases every year, the Indian books for about 80% of all zooanthroponosis VL cases worldwide.3 VL, on the other hand, is significantly underreported, with stated coverage varying not just among nations but also within districts inside a country.3 India has the greatest national VL incidence in the world, followed by Bangladesh and Nepal. In these three countries, the people at risk of contracting VL totals almost 300 million people. VL data for other south Asian nations like Sri Lanka and Bhutan on the other hand, is scarce, with the prevailing idea that it is sporadic and scattered being upheld in the majority of VL infection.3 PKDL is a fascinating clinical situation that has been defined in India and occurs in 10% of VL cases following deceptive VL cure. Patients with PKDL have skin lesions and are thought to be sources for VL transmission, while the significance of PKDL is unclear.27 In other south Asian nations, Leishmania donovani is a well-established human VL agent; however, dermo-tropism, which is common in Sri Lanka, has only been reported on rare occasions in other places.21,34 This has made investigators to search on the involvement of protozoal parasite58,75 and human or animals genetics in determination of phenotype of disease.54
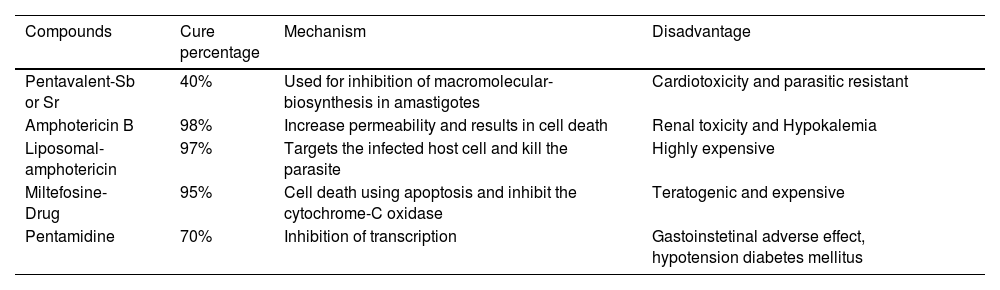
Current treatment for leishmaniaThe available treatment for leishmaniasis is far from ideal. In India, resistance to antimonials, which was prescribed for VL and CL treatment from more than 40 years,26,67 is now common. Even though new medications for VL treatment of have approved in India, such as AmBisomew amphotericin B, and oral medication Miltefosine, the treatment challenges remain the same due to cost and side effects.65 Bisphosphonates and plant compounds have been shown to be active in contradiction of experimental model infections. Therefore, the search for new medications continues (Singh et al., 2016). In biochemical and molecular investigations, many prospective therapeutic targets have been identified, and some have been verified.63 Attempts are being made to exploit these targets. Because there is no vaccine for VL, it is only possible to tackle it with chemotherapy. Due to problems with efficacy, side-effects, developing drug-resistance, extremely-high cost, and the requirement for hospitalization to take the complete course of medication, the available dealing choices for VL are limited and do not meet sufficient criteria. Numerous clinical trials have been conducted in India to improve therapy routines and guard the efficacy of the few anti-leishmanials that are now accessible (Om et al., 2016). Certainly, there have been significant recent advancements in the field of VL therapy, and if adequately implemented, this illness might be eradicated from most endemic portions of the globe. Table 1 lists of anti-leishmanial drugs available for VL and their significant safety, stability, and cost issues.59
Anti-leishmanial drugs available for VL.
The handling of VL patients is intricate by the patient's late appearance at a progressive stage of sickness, and the success of treatment is largely determined by drug-parasite interaction and the human host.61 The most disturbing reports came from Bihar (India), where 75% of VL patients were found to be insensitive to SBV treatment, though reports from Nepal specify a rate of up to 25% unresponsiveness in a district.6250 Multiple SBV resistance mechanisms have recently been found in the Indian subcontinent,18 including metal loss, thiol over-expression, multidrug resistant trailer, and condensed drug absorption due to reduced expression of drug-aquaporins in several investigational settings.14 Many other findings on drug-resistance were identified, including the role of transporters of efflux, changes in the level of thiol which are intracellular drug-resistant explored in clinical isolates. AmB resistance was linked to changes in the profile of sterol in the parasite membrane, where ergosterol is replaced by cholesta-5,7,3-ol, lowering the parasite's affinity for the medication. Proteomic study revealed insight on the participation of energy pathways that are increased, such as the glycolytic cycle, as well as the necessity of scavenging pathways and heat shock protein as supplementary weapons for safety against the drug-induced strain.11 Miltefosine resistance is a major worry because it is the only oral antileishmanial medication currently available.16 The elements lashing the parasite machinery for adaptation in counteraction of drug-induced pressure has been considered to be incomplete therapy and the drug's long half-life in the circulation. Aside from the aforementioned factors, enhanced drug efflux has been identified as a potentially dangerous source of drug resistance.16 Paromomycin, a medication that targets the parasite's mitochondrial system, has resulted in the parasite emerging as a robust survivor, with condensed drug binding to the surface, high translational activities, and high expression levels of ACB transporters.12 Inadequate treatment, either in terms of regimen or duration, is regarded to be the primary cause of treatment failure and the establishment of drug resistance.51 In the occurrence or absenteeism of drug burden, there is no information regarding the subtleties of drug-resistant Leishmanial populations. As a result, drug effectiveness monitoring and fast reporting are critical to bringing curative steps to drug procedures. This necessitates the usage of tools, as well as a structure for implementing them (Enanmat, 2001).
Despite significant scientific advancements in the extensive field of leishmaniasis over the last decade, including genomic sequencing of multiple organisms producing diverse forms of leishmaniasis, they have had no influence on the excellence of clinical upkeep for VL in the field because of the availability of very inadequate number of anti-leishmanial medications. It is critical to maintain the efficacy of these medications to treat patients and control VL. This will necessitate the continuous provision of quality medications, the advancement of treatment adherence, and the regulation of treatment efficacy as well as drug resistance. Monotherapy with anti-leishmanial medications has a high chance to produce resistant parasites, hence multidrug treatment is suggested.61 Another significant issue is relapse after medication treatment, and currently there are no clear analytical markers to predict people who are likely to fail medication treatment. Because our limited understanding of the processes underlying the establishment of resistance to drugs, its subtleties, and the influence of new medication, and there are no proven tools for monitoring treatment effectiveness under normal conditions, new measures for assessing treatment success and drug resistance are urgently needed to support the VL eradication program's medication policy.
Leishmanial key major surface protease, GP63Despite significant progress in the expansion of antibiotics, parasitic infections remain a serious danger to global health. For a variety of explanations, including the complicated interactions of host–parasite, investigation of new medicines for this illness is severely impeded. Furthermore, drug development is hampered by lower mortality rates related to some other infectious diseases, owing to the parasites' evolutionary approach to keep their hosts active. Finally, and perhaps most prominently, the statistics that these diseases are largely widespread in poor nations, which gets no attention from pharmaceutical firms, has led to few researches on field. When one considers that there is currently no vaccination available for any parasite disease produced by protozoans, helminths, or nematodes, the urgency of the problem becomes clear. Furthermore, many of the present treatments for these disorders have serious adverse effects, and resistance is rapidly spreading. Parasites benefit from a variety of virulence features that let them to avoid and overpower the host immune system, letting them to stay in the host body for long periods of time, if not permanently. The majority of parasite illnesses research have been devoted to identifying these factors and seeking to prevent them from acting.38 Many virulence factors have been found to far, with many of them being similar across species, however some are both species and strain specific. Certainly, findings from complete genome sequence analyses thus far agree with this virulence component distribution and with more in-depth studies. Proteases are a vast category of parasitic virulence factors that are shared – through comparable activity – by a variety of parasites both nearby and far apart. Various investigations have revealed that leishmania proteases, particularly the main surface metalloprotease GP63 and the cysteine proteases, are required for the formation and persistence of leishmania infection. Previous research has demonstrated that GP63, which is abundant on the surface of promastigote leishmania parasites, is critical for the parasite's ability to avoid and survive complement-mediated lysis prior to macrophage internalization. These experiments also revealed that GP63 can cleave the complement component c3b into its inactive form c3bi. GP63 can also break some of the host cell's extracellular matrix proteins, which helps the parasite move about inside the connective tissue.74 Recently, it was discovered that leishmania GP63 may quickly penetrate the host macrophage's intracellular milieu and activate host protein tyrosine phosphatases (PTPs).24 PTP activation was discovered to dephosphorylate and inactivate numerous essential signaling kinases engaged in biochemical cascades, allowing phagocytes to perform crucial microbicidal tasks in innate immune retorts. The parasites are able to establish infection by making the host cells inactive in this way. It was also discovered that the L. major parasites that lacks the GP63 were powerless to trigger the PTPs, which results in the shutdown of macrophage signaling. Because of the loss of GP63, the parasites provoke innate inflammatory responses, which severely limited their capability to start and uphold the infection.72 Surprisingly, despite being a high virulence factor of leishmaniasis, GP63 has received little attention from drug-development initiatives. Although the biochemistry and construction of GP63 have been extensively investigated, researchers have only recently started the designing of PI of this protease. For example, Corradin et al. developed peptide inhibitors grounded on MARCKS, one of GP63′s target proteins. However, they were unable to show that these inhibitors significantly reduced leishmania infectivity.13 As a result, there is still a lot of room for finding and designing strong inhibitors of GP63 specifically to stop or minimize leishmanial infections by favoring activation of the macrophage system.
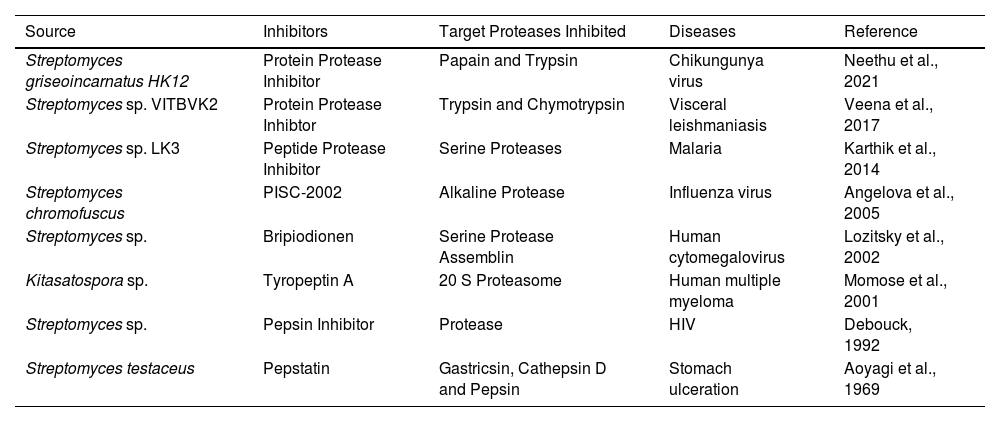
Role of protease inhibitors as anti-microbial agentsProtease inhibitors are widely regarded as one of the most effective HIV treatments ever devised. Antiretroviral therapy includes the use of protease inhibitors. Proteases are required for the reproduction of some viruses, such as HIV. As a result, protease inhibitors are being used as an antiviral medication. The growing AIDS epidemic and the disease's unrelenting character have fueled the search for antiviral medication to stop HIV, the disease's causal agent, from replicating. The HIV-1 protease is an enzyme that is essential for viral replication. As a result, inhibitors of this protease enzyme are useful in avoiding infection. A streptomyces pepsin inhibitor found to be an effective HIV-1 protease inhibitor.17 Baba et al.8 also limit HIV-1 replication. Bripiodionen was shown to be particularly effective against human cytomegalovirus proteases after being isolated from Streptomyces sp. S. chromofuscus, isolated from black soil, developed an extracellular protease inhibitor with alkaline phosphatase activity and a protease inhibitor from Streptomyces sp. suppresses influenza virus propagation.36 In a mice model of experimental influenza H3N2 virus infection, it likewise exhibited a strong protective effect.4 With the rise of HIV, leishmania coinfection in some parts of the world, the idea of using HIV protease inhibitors to treat leishmania has piqued people's curiosity. Three of the PI used in extremely active antiretroviral therapy for HIV also aims the parasite, as per Savoia et al., who were then trailed up by Santos et al. These parasite inhibitors significantly reduce leishmania parasite growth and internalization in host macrophages, despite no deceptive inhibition of the major surface protein.55 Proteolytic activity in bacteria was regulated by naturally occurring protease inhibitors. They are divided into two broad classes built on the basis of physical dichotomy: peptidomimetic inhibitors and protein PI. Protease inhibitors have also been found to be effective against other protozoan-related infectious illnesses. Inhibitors of cysteine protease were used for treating disease, which is triggered by T. cruzi. A cysteine PI aiming cruzain, the primary T. cruzi protease is one of the most promising novel therapeutic led for Chagas disease. The immunodeficient animal was saved from a deadly Chagas infection after being treated with vinyl sulfone protease inhibitors.20 Karthik et al.30 discovered a protease inhibitor (peptide) in deep-sea Nicobar sediment samples from marine actinobacteria. This inhibitor has been shown to be effective against the malaria parasite P. falciparum. Apart from this, protease inhibitors also play a major role as a therapeutic agent in many diseases (Table 2).
List of protease inhibitors from marine actinobacteria as a therapeutic agent in various diseases.
Marine actinobacteria have long been regarded as a gold mine in terms of their secondary metabolite potential. Nadson40 isolated marine actinobacteria from salt mud for the first time. Baam et al.7 from the Bombay maritime waters published the first report on marine actinobacteria isolation in India. A variety of natural compounds have been studied in relation to marine microorganisms. The properties of marine actinobacteria differ from those of their terrestrial counterparts. Actinomycetes, principally from the genera Streptomyces are known to produce about 70% of the world's drugs. A soybean plant produced the first known inhibitor.25 Later, in 1962, Hoyem and Skulberg reported the discovery of the first trypsin inhibitors from Clostridium botulinum types A, B, and E. From Streptomyces sp., Umezawa6869 identified the initial low molecular weight inhibitor. Leupeptin was isolated from Actinomycetes sp. by Aoyagi et al. in 1969. Proteases like plasmin, trypsin, or papain, as well as thrombokinase and kallikrein, were all specifically inhibited by it. It reduces blood coagulation and has anti-inflammatory and anti-edema action. Leupeptin's structure has been characterized.5,32 It also prevents skin cancer in mouse injected by a single dose of 7,12dimethylbenz (a) anthracene, according to research.28 Umezawa et al.68 found chymotrypsin in culture filtrates of seven actinomycetes strains. For screening bacteria that produce protease inhibitors, a double agar layer method was proposed. The prevalence of such organisms in mud, dung, and grass samples was investigated. A considerable protease inhibitory capacity was found in over 10,000 colonies. S. violascens was found to be an organism with significant trypsin inhibitory capability. For the first time a protease inhibitor screening method was published.64 Up until now, more than 100 inhibitors from Streptomyces sp have been reported. Most of the species are known to produce antipapain that can inhibit papain enzymes, cathpesin B and trypsin. Similarly, another study on S. hygroscopicus and S. lavendulae revealed the production of chymostatin against chymotrypsin and elastatinal from S. griseoruber60; Umezawa et al., 1972). Phosphoramidon produced by S. tanashiensis inhibited the metallo proteases such as thermolysin and metallo-endopeptidases of Bacillus subtilis and S. griseus.31S. noboritoensis was capable of producing elasnin inhibitor against human granulocyte elastase.47,41 Similarly, Odibo et al. (2011) reported β-microbial alkaline protease inhibitor from streptomycetes which inhibits chymotrypsin and thiol proteinases. S. mazunensis was capable of producing talopeptin.22 Ogura et al.46 reported streptin from S. tanabeensis which was capable to inhibit calpain, apain, trypsin and other proteases. This was the first report on serine and thiol protease inhibitors from Streptomyces sp. Imada in29 reported many enzyme inhibitors like pyroglutamyl peptidase, α-amylase inhibitors and glucosidase which were extracted from marine streptomyces.45 Novel protease inhibitor, PISC2002 was extracted from the supernatant of S. chromofuscus.4 PISC2002 was known to have activity against trypsin and proteinase and no activity against cathepsin and D chymotrypsin. A new cytotoxic antibiotic was discovered by testing the culture filtrates for inhibition of tumor growth in vivo or tumor cell growth in vitro by the inhibition of macromolecular synthesis or inhibition of certain specific enzymes such as reverse transcriptase, glyoxalase, adenosine deaminase and tyrosine protein kinase. Woo et al.73 was found deoxycoformycin in S. antibiotics by screening of adenosine deaminase inhibitors. Glyoxalase inhibitor was isolated from S. filipinensis (Frederick et al., 2000). It suppressed Yoshida rat sarcoma cells in tissue culture at IC50 18 µg/ml and Ehrlich carcinoma in mice was suppressed by daily intraperitoneal injection of 10 mg/kg for 10 days. Revistin was isolated from streptomyces culture filtrates and it is acting against reverse transcriptase.44 Following this, Nishio et al.43 discovered two inhibitors against reverse transcriptase from S. filipinensis such as streptonigrin and retrostatin. Also, reported limocrocin and chromostin from S. limosus and unidentified actinomycete respectively. Tunac and Underhill66 found 2’-chloropentostatin from culture broth of Actinomadura sp ATCC3965. Subsequently, adechlorin was found in other Actinomadurae.48 Omura et al. (1986) found Adecypenol from Streptomyces sp. OM-3223. These three compounds were inhibiting the adenosine deaminase. Sathish and Bhaskar, (2018) reported the activity of alpha glucosidase inhibitor from marine actinobacteria for the regulation of raised blood sugar level in swiss albino mice33. In a very recent report, marine Streptomyces sp. showed promising activity (protease inhibitor) against pure proteases like chymotrypsin and trypsin against the protease isolated from P. aeruginosa (Neha et al., 2020). Hence, extractions of potential protease inhibitors from marine organisms are of greater importance for the discovery and development of new drugs against infectious diseases.
In conclusion, protease inhibitor drug bioproduction with a concentration on leishmanial major surface proteases or zinc-metalloproteases will be quite effective in controlling leishmania species pathogenesis. In light of the scarcity of anti-leishmanial drug studies focusing on major surface protease and protease inhibitors from marine actinobacteria targets such crucial proteases could provide the starting point for the discovery of effective medications to treat visceral leishmaniasis. Advances in vaccine development and treatment could prevent substantial morbidity and mortality from this disease.
No funding sources.