Perianal tuberculosis is extremely rare without previous or active pulmonary infection. Ulcerative skin lesion is a rare presentation of extrapulmonary tuberculosis in the oral, perianal, or genital mucosa and the adjacent skin.
Case reportA 71-year-old woman complained of pain during evacuation and fecal incontinence for two years. There was an ulcerated lesion in the perianal and intergluteal region and perianal fistulous tracts. A polymerase chain reaction test on blood and biopsies of perianal ulcers, perianal fistula, and the intergluteal area was positive for Mycobacterium tuberculosis. The pathological examination revealed a chronic epithelioid granulomatous inflammatory process with the presence of multinucleated giant cells. After the end of the tuberculosis drug regimen, there was marked improvement in the patient's clinical condition.
ConclusionEven in the absence of an identifiable primary focus, tuberculosis should be considered in the differential diagnosis of ulcerative and fistulous lesions of the perianal area.
Tuberculosis (TB) is an infectious granulomatous disease caused by Mycobacterium tuberculosis, an alcohol-acid-resistant bacillus transmitted primarily to the respiratory system.1 According to the World Health Organization, 20% to 40% of the world's population has already been infected by M. tuberculosis, with an estimated 8-9 million new cases each year.1 Despite improvement in public health policies and the availability of effective antibiotics, TB remains a significant health problem all over the world, especially in low-income countries.1
Pulmonary disease is the most frequent manifestation of TB, but extrapulmonary TB accounts for at least 15% of all cases.1 Extrapulmonary dissemination mainly consists of involvement of the pleura (26%), lymph nodes (17%), genitourinary tract (15%), bones and joints (14%), meninges (6%) or peritoneum (4%), and as miliary TB (8%). In comparison, disease of the intestinal tract constitutes less than 1% of the extrapulmonary forms of the disease.1
TB can involve any part of the gastrointestinal system, such as the stomach, duodenum, ileocecal region, colon, rectum, or anus.2 The most frequently involved part of the intestinal tract is the ileocecal region.2 Less than 1% TB cases manifest as gastrointestinal TB, and perianal TB is much less frequently encountered, 1% of all cases of TB of the digestive tract.2
Ulcerative cutaneous TB, also known as TB cutis orificialis, is a rare manifestation of cutaneous TB characterized by painful ulcerative lesions that affect the mucous membranes and the skin adjacent to the orifices.3 The tongue is the most frequently affected site, but the perianal area can also be affected.3,4 Perianal TB is a sporadic presentation of cutaneous M. tuberculosis infection, representing 0.001% of all extrapulmonary TB cases; it usually manifests in the 4th decade of life and is more commonly found in men.2,4 The anogenital region can be affected by the hematogenous spread of M. tuberculosis.3,4 To the best of our knowledge, fewer than 60 cases of perianal TB have been reported in the literature, and active pulmonary TB was observed in only half of the cases described.4,5
Case reportThis case report followed CARE guidelines.
A 71-year old female patient, white homemaker, married, born and residing in Santo André (São Paulo, Brazil), reported pain during evacuation, fecal incontinence, and wounds in the perianal and intergluteal region for two years, associated with weight loss of 20 kg in the last year. She reported daily bowel movement, without evacuation effort, with neither blood nor mucus in her stool. The patient had no cough, afternoon fever, or night sweats. The patient was hypertensive, no previous surgeries, and had no history of family members with TB or contact with people with TB. The patient had a history of eight normal childbirths. There was a slight pallor on physical examination, with no other abnormalities. Her BMI was 31. The proctological inspection showed a lesion in the intergluteal region, ulcerated and hyperemic, with raised borders, 10 cm in length, indurated, and intensely painful to touch. In the perianal area, there were deep circumferential ulcers that affected the anterior and posterior quadrants of the anal circumference and fistulous perianal tracts with an outflow of seropurulent secretion. The rectum was intensely painful to the touch. During the colonoscopy, the device progressed to the cecum with visualization of the cecal ileum valve and terminal ileum without alterations, but there was pancolonic diverticular disease.
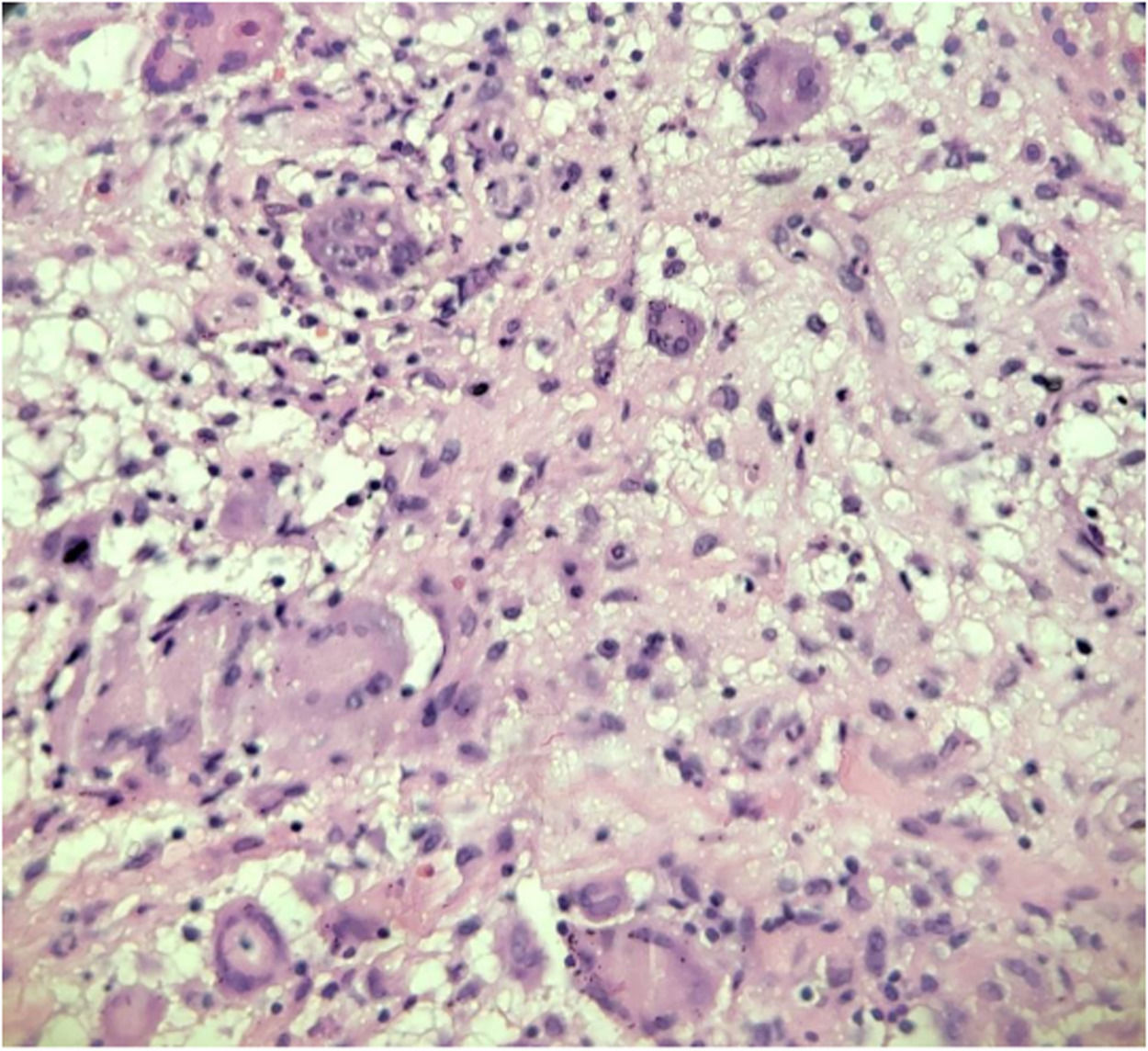
An abdominal and pelvic ultrasound was normal. Chest radiography, computed tomography (CT) of the abdomen and chest, and abdominal and pelvic magnetic resonance did not show relevant alterations. The purified protein derivative (PPD) and sputum alcohol-resistant bacilli tests were negative, as was the serology for HIV detection. However, the polymerase chain reaction (PCR) test on blood was positive for M. tuberculosis. The patient underwent a proctological examination with narcosis. Anoscopy revealed the presence of ulcers up to 2 mm in length, with stenosis 1cm from the anal edge and deep ulcers in the anterior and posterior region of the anal canal with exposure of the sphincter muscle. Biopsies of the intergluteal lesion and anal canal ulcers and a first-time fistulotomy using seton were performed. The anatomopathological examination of biopsies of the perianal region and the intergluteal lesion revealed a chronic granulomatous inflammatory process with mucosal ulceration, and tests for alcohol-acid-resistant fungi and bacilli were negative. The patient underwent a new proctological examination with narcosis and a second stage of fistulotomy. We performed biopsies of the anal fistula tract and perianal tissue. The pathological analysis (Fig. 1) revealed an epithelioid granulomatous process with multinucleated giant cells and granulation tissue in the intergluteal sulcus and the squamous tissue obtained from the perianal fistula tract.
Tests for fungi using Grocott-Gomori staining and for alcohol-acid-resistant bacilli using Ziehl-Neelsen and Fite-Faraco stains and a culture for M. tuberculosis were negative.
PCR is a simple and rapid test used for detection of M. tuberculosis because it amplifies a DNA sequence which is unique to mycobacteria (15). Pretreatment of tissue samples from biopsies of the intergluteal lesion and fistula tract was performed using the standard preparation method for laboratory diagnosis of mycobacteria (2.5% N-acetyl-L-cysteine-NaOH solution and decontamination by Kirchner's solution). Perineal tissues were immediately incubated at 80°C for 10 minutes. Then the samples were concentrated by centrifugation at 5000 × g for 5 minutes. The pellet was used for DNA extraction. Total DNA was extracted from the samples using Taqman™ Real kit -Time PCR Assays (Thermo Fisher Scientific, Waltham, MA, USA) according to the procedure provided in the kit protocol. The DNA pellet was reconstituted in 20 µL of distilled water and stored at -20°C. The assay detects a 123 bp region of the M. tuberculosis complex-specific insertion sequence IS6110. Briefly, PCR amplification was performed to detect M. tuberculosis using a direct primer (5′-CCT GCG AGC GTA GGC GTC GGT-3′) and primer reverse (5′-CTC CTC CAG CCC CCC CTT CCC-3′). PCR reactions were performed in a final volume of 25 µL containing 10 mM Tris-HCl (pH 8.3), 50 mM KCl, 2.5 mM MgCl2, 0.01% gelatin, 0.2 mM (each) of deoxynucleoside triphosphate, 1 mM each of forward and reverse primers and 1 U of Taq DNA polymerase. The PCR system 2700 thermal cycler (Applied Biosystem Inc, Foster City, CA, USA) was programmed to initially incubate the samples for 2 min at 94°C, followed by 35 cycles consisting of 94°C for 45 seconds, 53°C for 45 seconds and 72°C for 1 minute. The final extension was given at 72°C for 10 minutes. The specific detection of M. tuberculosis DNA was identified by specific bands of 123 bp of DNA on a 2% agarose gel, stained with ethidium bromide, and evaluated in a UV transilluminator. PCR product sizes were estimated according to the migration pattern of a 100 bp DNA ladder. The PCR test for M. tuberculosis in the intergluteal tissue was positive, but this test was negative in the material from the fistula tract.
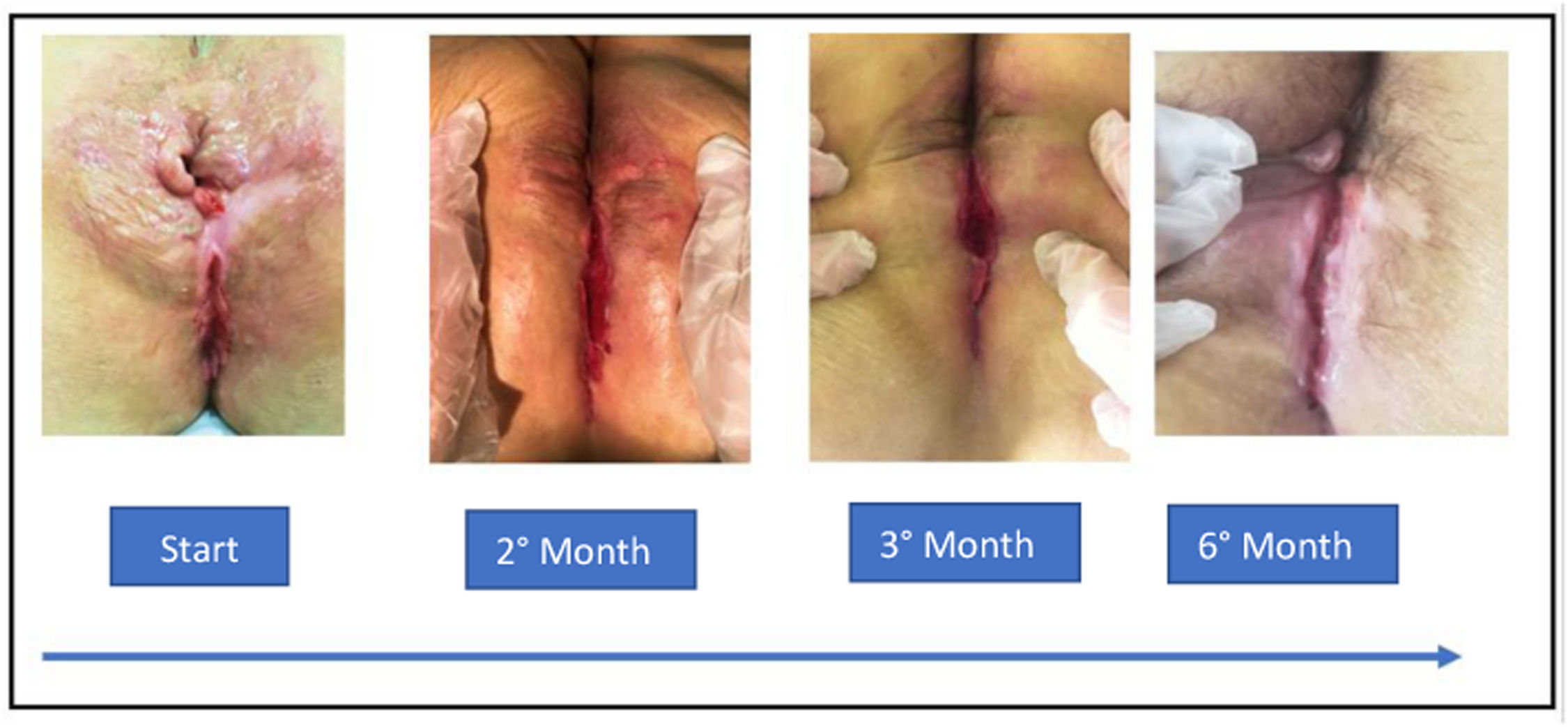
The patient was treated with the RIPE regimen (isoniazid, rifampicin, ethambutol, and pyrazinamide). However, she discontinued treatment after 2 weeks due to the appearance of nausea, vomiting, and erythema throughout the body. After this clinical picture improved, we reintroduced the RIPE regimen for 6 months. After the end of the tuberculostatic regimen, the patient's examination revealed that the intergluteal fold had granulation tissue and no secretion (Fig. 2). The rectal examination was painless, with no fistulas or fissures. The patient maintains effortless evacuation of pale stools 2 to 3 times a day without proctological complaints.
DiscussionPerianal TB usually occurs secondarily to or coexists with a lung lesion.5 However, the patient had no identified pulmonary focus in the present case.
In addition to the hematogenous dissemination of a pulmonary focus, causes of perianal TB include the ingestion of sputum containing active pulmonary disease bacilli, lymphatic dissemination of intestinal or genitourinary disease from regional lymph nodes5,7,8 or even direct propagation from adjacent organs.2 Of these possible mechanisms, the ingestion of bacilli in sputum is the most frequent.2 It was not possible to identify another focus of TB in the patient herein reported.
Perianal TB can manifest with local pain, mucopurulent secretion, and multiple complex perianal fistulas,9,10 which we also observed in the case described here. These fistulas can persist and have a prolonged and relapsing course despite proper surgical management.11 Inguinal adenopathy, perianal ulceration with purulent exudates, anal stenosis, anal ulceration, and fissures have also been reported.11,12 The patient we studied had perianal ulceration with purulent exudates and anal stenosis and ulceration.
Clinically, perianal TB can manifest macroscopically in ulcerative, verrucous, or lupoid forms.12 Ulcerative TB is the most common type, with well-defined borders, usually secondary to a focus in the lungs or intestine.12 The verrucous type tends to extend to the anal region from the perianal area with a development pattern similar to that of a wart. Lupoid TB occurs secondarily to TB in other body parts; it starts as a small, round nodule, reddish-brown and somewhat soft. Gradually, a well-defined ulcer develops in the center of the nodule. The perianal TB of the patient in this report manifested as the ulcerative form, although the primary focus of TB was not identified.
TB is also related to immunocompromised states, as the infection occurs in cases of HIV infection and leprosy,13 which were not observed in the patient.
The diagnosis of perianal TB depends, in addition to clinical manifestations, on the performance of a skin tuberculin test (PPD or Mantoux reaction), M. tuberculosis smear and culture, histopathological examination of the lesion, history of TB in other systems or organs, and a PCR test.1,14
PPD is positive in 75% of cases, but it can be negative in patients with perianal TB14 as it was the case in the patient in this report due to decreased host defense against TB. A culture for M. tuberculosis from the seropurulent secretion from perianal fistulous tracts and tissue of these fistulas and from intergluteal sulcus was negative. Although the gold standard for the diagnosis of TB recommended by the WHO is the use of culture, the proportion of disease with negative culture ranges from 15% to 20%, even in patients with pulmonary TB. These patients are less likely to have a cough, weight loss, and pulmonary cavitation, as it was the case in the present patient. This presentation may occur because the mycobacteria are more contained in the granulomatous tissue or may no longer be viable due to the reaction of the tissue in which these bacteria are found and the patient's immunity.15,16 Only negative culture can rule out TB in suspected cases with negative direct smears and PCR tests.16
Histopathological findings usually include ulceration surrounded by a nonspecific inflammatory infiltrate and extensive caseous necrosis.14 Granulomas composed of epithelioid and Langhans-type giant cells can also be seen in the dermis.14 The biopsy of the intergluteal region of the patient in this report revealed a chronic granulomatous inflammatory process, and the biopsy of the perianal fistula tract also showed the presence of an epithelioid granulomatous process with the presence of multinucleated giant cells (Langhans cells), although in the absence of caseous necrosis. Alcohol-acid-resistant fungi and bacilli were negative in both biopsy materials.
The detection of mycobacterial DNA by PCR in clinical samples contributes to the rapid diagnosis of TB infection.14–16 This minimally invasive test can detect bacterial DNA within 48 hours with high sensitivity and specificity. In this report, the patient had a positive PCR test for mycobacterial DNA in peripheral blood and tissue obtained from perianal fistula material.
PCR has the highest sensitivity (79.4%), followed by histopathological examination (73.5%), cultures (29%-47%), and smear of material from the perianal region (5.8%).16
The differential diagnosis of TB ulcerative lesions in the perianal region includes Crohn´s disease, pyoderma gangrenosum, anorectal abscesses associated with mixed flora, cutaneous amebiasis, sarcoidosis, syphilis, lymphogranuloma venereum, neoplasms, hidradenitis, sarcoidosis, herpes simplex, deep mycoses (actinomycosis), lupus vulgaris, erythema induratum of Bazin, and foreign body reactions.7,8,17 Of these diseases, Crohn's disease and pyoderma gangrenosum are the most important.2,12 Perianal TB and Crohn´s disease are similar in that they usually present with ulcerative lesions and ulcerated plaques and granulomas on histopathological examination.2,7,8,14 Perianal TB can be differentiated from Crohn´s disease by Ziehl-Neelsen staining, culture for M. tuberculosis, and, particularly, the PCR test for TB DNA,14–16 as shown in the patient herein reported. Pyoderma gangrenosum is a rare ulcerating neutrophilic dermatosis that may rarely involve the perineal region. It often begins as pustules, erythema, and blisters, rapidly expanding in a centrifugal pattern to form a well-defined ulcer. The etiology of pyoderma gangrenosum probably involves an interplay of genetic and environmental factors with loss of innate immune regulation and altered neutrophil chemotaxis. Pyoderma gangrenosum may even respond to antibacterial drugs with an immunomodulatory effect, such as rifampicin.6Once the diagnosis of perianal TB has been established, a thorough search for the origin and the involvement of other sites in the body in TB should be undertaken.14 In addition to chest radiography and CT scans, patients should also be evaluated with a barium enema, ultrasound, CT and magnetic resonance imaging of the abdomen, and colonoscopy to identify possible gastrointestinal and peritoneal involvement.14 The patient in this report underwent chest x-rays and CT scans that did not reveal changes from pulmonary TB, in addition to a pelvic MRI, which was normal. It should be borne in mind that perianal TB cases may arise as an incipient disease without any previous or active lung infection.14–17
Treatment can be both medical and surgical.18 Perianal TB does not resolve spontaneously and can lead to death by miliary spread if not properly treated.14,18
Ulcerative lesions of the anus associated with perianal TB usually regress within a few weeks after clinical treatment.19 Antimicrobial susceptibility testing should be performed to select the best treatment option for each case to eradicate TB and prevent the emergence of resistant cases.8,14 However, if the skin lesions do not respond to medication or are accompanied by obstruction or abscess, a surgical approach is necessary.19
Review of 58 cases of perianal TB in the literature by Tago et al.4 found that the duration of the persistent perianal lesion was significantly longer in patients without active pulmonary TB than in those with active pulmonary TB. Thus, TB should be considered in cases of non-healing or recurrent perianal lesions.
In conclusion, as it is an infrequent etiology, the diagnosis of perianal TB is challenging, especially in the absence of a pulmonary focus. TB should be considered in the differential diagnosis of perianal ulcers and fistulas, mainly in non-healing and recurrent anal lesions, especially in regions where TB is endemic. Treatment with anti-TB agents can provide complete recovery.