HIV infection may be considered a chronic condition for people living with HIV with access to antiretrovirals and this has effectively increased survival. Moreover, this has also facilitated the emergence of other comorbidities increasing the risk for drug–drug interactions and polypharmacy. The profile of these interactions as well as their consequences for people living with HIV are still not completely elucidated. The objectives of this study were to describe the profile of these interactions, their prevalence and their classification according to the potential for significant or non-significant drug–drug interactions. From June 2015 to July 2016, people living with HIV on follow-up at an Infectious Diseases Referral Center in Belo Horizonte, Brazil have been investigated for the presence of drug–drug interactions. A total of 304 patients were included and the majority (75%) had less than 50 years of age, male (66.4%), and 37.8% self-defined as brown skinned. Approximately 24% were on five or more medications and half of them presented with drug–drug interactions. Patients older than 50 years had a higher frequency of antiretrovirals drug–drug interactions with other drugs compared to younger patients (p=0.002). No relationship was found between the number of drug–drug interactions and the effectiveness of antiretrovirals. As expected, the higher the number of non-HIV medications used (OR=1.129; 95%CI 1.004–1.209; p=0.04) was associated with an increase in drug–drug interactions. The high prevalence of drug–drug interactions found and the data collected should be useful to establish measures of quaternary prevention and to increase the medication security for people living with HIV.
The Human Immunodeficiency Virus (HIV) infection may now be considered a chronic condition. As a result, HIV-related mortality has been greatly reduced, and the life expectancy of HIV-infected individuals is near normal.1 Nonetheless, it can lead to precocious aging and to the emergence of other comorbidities with possible risks of harmful drug interactions. A major challenge is thus polypharmacy and, consequently, drug–drug interactions (DIs). In humans, cytochrome P450 (CYP) has a central role in phase I drug metabolism where they are of critical importance to two of the most significant problems in clinical pharmacology: drug interactions and inter-individual variability in drug metabolism.2 Therefore, medications to treat comorbidities, supplements, and legal and illegal drugs that inhibit or induce CYP can cause damage and toxicity to the body.3 The basic hypothesis of DIs is based on the interference of drug's bioavailability that ARV may cause,4 which must be detected for increasing drug safety for people living with HIV (PLWH).1 The aims of this study were to describe the profile of DIs between antiretrovirals (ARVs) and other medications, evaluate their prevalence, and to classify DIs as potentially significant or non-significant.
MethodsThis was an observational study conducted from June 2015 to July 2016 involving HIV-infected adult patients followed at a Reference Center for Infectious Diseases in Belo Horizonte city, Southeast Brazil. This center is a public health outpatient clinic which provides medical care and ARVs through the Brazilian public health system.
Sample size calculation was based on the prevalence of drug interactions with ARVs described in the international literature (69.9%).5 Considering an average number of adult patients followed per year (4000) with a confidence level of 95% and an absolute precision of 0.05, a minimum sample of 304 patients was required. After being presented the scope of the study, consenting patients were enrolled and interviewed on the same day of medical consultation. Medical records and Ministry of Health HIV databases were used to collect demographic data (age, sex, skin color and employment status) as well medical records (ARVs regimen, non-ARV drugs, CD4+ count and HIV viral load (VL). A clinical pharmacist was in charge of collecting the patient self-report and the medical prescription history for the previous 12 months. CD4+ count was categorized as <350, 350–500, and >500cells/mm3 and viral suppression was defined as a VL less than 40copies/mL.
The Research Ethics Committee of the Federal University of Minas Gerais approved this study under the number CAAE 50837015.7.0000.5149.
Clinically significant drug–drug interactions interactions (DIs)×potential interactionsWe used the comprehensive University of Liverpool HIV drug interactions database,6 which aggregates published findings mainly from European and North American studies. Two levels of interactions were considered: contraindicated or with high evidence of interaction with concurrently prescribed ARVs (clinically significant interactions) or as having moderate evidence of interaction with concurrently prescribed ARVs (potential interactions). Only concomitant prescription of ARVs medications with non-ARVs medication (termed from here on “ARV/non-ARV”) were considered.
DIs considered as contraindicated or with high evidence of interaction with concurrently prescribed ARVs meant that these drugs should have not been co-administered as it may lead to sub-therapeutic ARVs blood levels and treatment failure due to reduced absorption and/or increased metabolism.4 Likewise, this type of DIs can lead to supra-therapeutic levels of ARVs or co-administered drugs that are metabolized by the same enzymatic pathway.7
The classification of DIs, clinically significant interactions (high evidence) or potential interactions (moderate evidence) were made available in the medical records to assist the multidisciplinary team on clinical decisions always aiming at patient safety.
All collected information was stored in a computerized database using Epi Data software version 3.1. The Statistical Package for Social Sciences (SPSS) software version 18.0 was used for the statistical analysis. To assess the prevalence of DIs, the number of interactions found was divided by the number of patients studied. Variables without normal distribution are described as median and interquartile range and non-parametric Mann–Whitney test (two groups) was used to compare the medians. Categorical variables were compared using chi-square or Fisher's exact test. Variables age and number of co-administered drugs were analyzed using the Mann–Whitney test. The categorical variables sex, ARVs regimen, last CD4+ count and last VL were analyzed using chi-square test. p-Values less than 0.20 in univariate analysis were included in the multivariate analyses, fitting the Poisson regression model with robust covariance matrix. The level of significance applied in this analysis was 0.05. The association between the selected exposure variables was estimated by Odds Ratio (OR) and 95% confidence interval (CI).
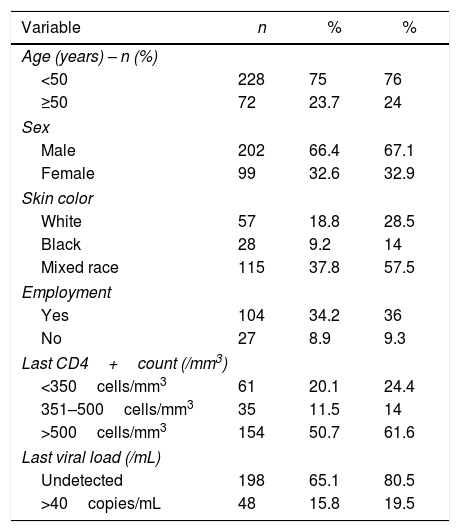
ResultsThree-hundred and four PLWH were included. Males comprised the majority of patients (66.4%); 75% were younger than 50 years. Baseline CD4+ count was higher than 500cells/mm3 in 50.7% individuals and 65.1% at baseline had VL count below 40copies/mL (Table 1). Four hundred and sixty-five DIs were found and 50% (152/304) had at least one DIs (Table 1).
Demographic and clinical features of the study population (n=304).
| Variable | n | % | % |
|---|---|---|---|
| Age (years) – n (%) | |||
| <50 | 228 | 75 | 76 |
| ≥50 | 72 | 23.7 | 24 |
| Sex | |||
| Male | 202 | 66.4 | 67.1 |
| Female | 99 | 32.6 | 32.9 |
| Skin color | |||
| White | 57 | 18.8 | 28.5 |
| Black | 28 | 9.2 | 14 |
| Mixed race | 115 | 37.8 | 57.5 |
| Employment | |||
| Yes | 104 | 34.2 | 36 |
| No | 27 | 8.9 | 9.3 |
| Last CD4+count (/mm3) | |||
| <350cells/mm3 | 61 | 20.1 | 24.4 |
| 351–500cells/mm3 | 35 | 11.5 | 14 |
| >500cells/mm3 | 154 | 50.7 | 61.6 |
| Last viral load (/mL) | |||
| Undetected | 198 | 65.1 | 80.5 |
| >40copies/mL | 48 | 15.8 | 19.5 |
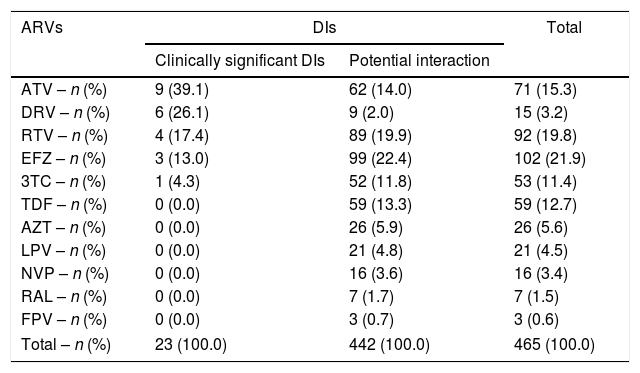
Efavirenz (EFZ)-based ARVs (21.9%) was the regimen that most frequently interacted with non-ARV drugs followed by ritonavir (RTV) (19.8%) and atazanavir (ATV) (15.3%). However, ATV-containing ARVs was the regimens with more DIs clinically significant (39.1%). Darunavir (DRV)-based regimens also presented a number of clinically significant DIs (26.1%) and only abacavir (ABC) and didanosine (ddI) presented no DIs (Table 2).
Drug–drug interactions in patients followed at a referral Infectious Diseases Service in Belo Horizonte, 2015–2016.
| ARVs | DIs | Total | |
|---|---|---|---|
| Clinically significant DIs | Potential interaction | ||
| ATV – n (%) | 9 (39.1) | 62 (14.0) | 71 (15.3) |
| DRV – n (%) | 6 (26.1) | 9 (2.0) | 15 (3.2) |
| RTV – n (%) | 4 (17.4) | 89 (19.9) | 92 (19.8) |
| EFZ – n (%) | 3 (13.0) | 99 (22.4) | 102 (21.9) |
| 3TC – n (%) | 1 (4.3) | 52 (11.8) | 53 (11.4) |
| TDF – n (%) | 0 (0.0) | 59 (13.3) | 59 (12.7) |
| AZT – n (%) | 0 (0.0) | 26 (5.9) | 26 (5.6) |
| LPV – n (%) | 0 (0.0) | 21 (4.8) | 21 (4.5) |
| NVP – n (%) | 0 (0.0) | 16 (3.6) | 16 (3.4) |
| RAL – n (%) | 0 (0.0) | 7 (1.7) | 7 (1.5) |
| FPV – n (%) | 0 (0.0) | 3 (0.7) | 3 (0.6) |
| Total – n (%) | 23 (100.0) | 442 (100.0) | 465 (100.0) |
Note: DIs, drug–drug interactions; ATV, atazanavir; DRV, darunavir; RTV, ritonavir; EFZ, efavirenz; 3TC, lamivudine; TDF, tenofovir; AZT, zidovudine; LPV, lopinavir; NVP, nevirapine; RAL, raltegravir; FPV, fosamprenavir.
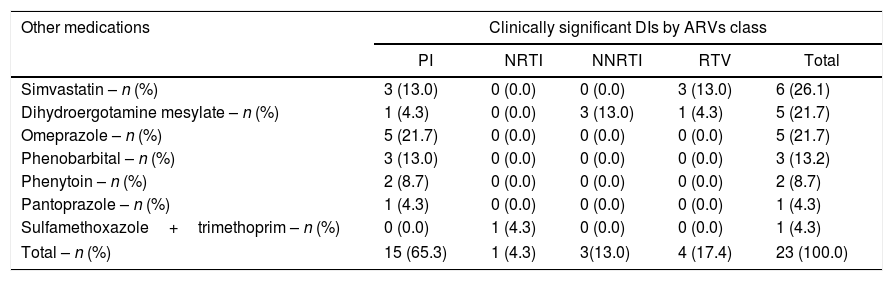
For the non-ARV drugs, sulfamethoxazole+trimethoprim (33.9%), benzodiazepines (25.8%), statins (17.7%), antidepressants (15.3%), lithium carbonate (5.7%), and drugs for erectile dysfunction (1.7%) presented DIs. Simvastatin was the non-ARV drug with more clinically significant interactions (26.1%), followed by dihydroergotamine mesylate (21.7%), omeprazole (21.7%), and phenobarbital (13%). Phenytoin (8.7%), pantoprazole (4.3%), and sulfamethoxazole+trimethoprim (4.3%) also presented clinically significant DIs, but with a lower frequency (Table 3).
Clinically significant drug–drug interactions between antiretrovirals classes and co-medications in patients with and without drug–drug interactions (n=304) followed at a referral Infectious Diseases Service in Belo Horizonte, 2015–2016.
| Other medications | Clinically significant DIs by ARVs class | ||||
|---|---|---|---|---|---|
| PI | NRTI | NNRTI | RTV | Total | |
| Simvastatin – n (%) | 3 (13.0) | 0 (0.0) | 0 (0.0) | 3 (13.0) | 6 (26.1) |
| Dihydroergotamine mesylate – n (%) | 1 (4.3) | 0 (0.0) | 3 (13.0) | 1 (4.3) | 5 (21.7) |
| Omeprazole – n (%) | 5 (21.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 5 (21.7) |
| Phenobarbital – n (%) | 3 (13.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 3 (13.2) |
| Phenytoin – n (%) | 2 (8.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (8.7) |
| Pantoprazole – n (%) | 1 (4.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (4.3) |
| Sulfamethoxazole+trimethoprim – n (%) | 0 (0.0) | 1 (4.3) | 0 (0.0) | 0 (0.0) | 1 (4.3) |
| Total – n (%) | 15 (65.3) | 1 (4.3) | 3(13.0) | 4 (17.4) | 23 (100.0) |
Note: DIs, drug–drug interactions; PI, protease inhibitor; NRTI, nucleoside reverse transcriptase inhibitor; NNRTI, non-nucleoside reverse transcriptase inhibitor; RTV, ritonavir.
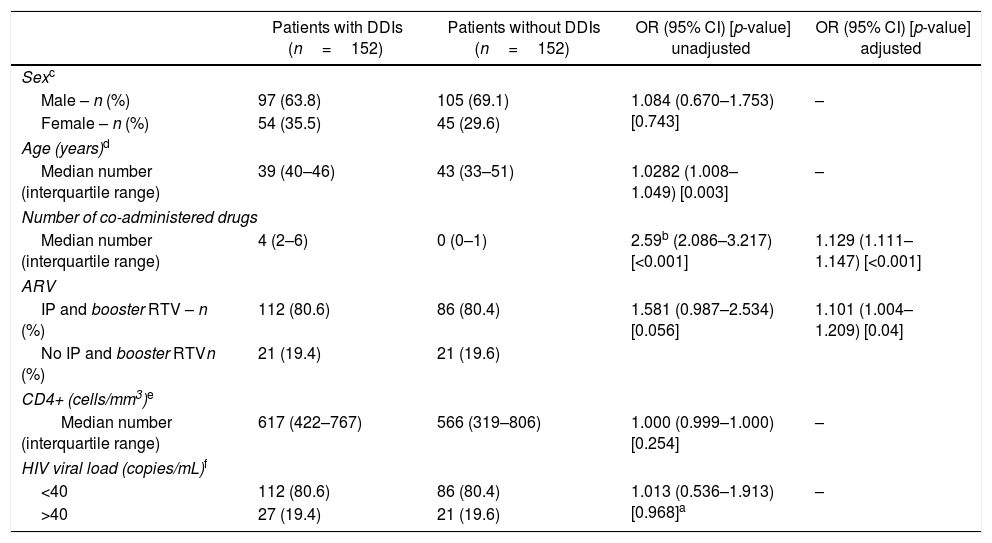
Regarding the number of drugs used, 96 patients (31.6%) had received only ARVs and 208 (68.4%) were on at least one non-ARV drugs. One hundred and seven patients (45.1%) were on four or more medications and 71 (23.3%) on five or more. Those older than 50 years had more drug interactions with non-ARV drugs (65.3%) compared to those younger than 50 years (45.9%). The age group was significantly associated with DIs between ARVs and non-ARV drugs (p=0.002).
In multivariate analysis using the Poisson regression model with robust covariance matrix, the number of co-administered drugs and use of protease inhibitor (PI) and RTV were considered independently associated with drug interactions (OR=1.129; 95% CI=1.111–1.147; p=0.001) and (OR=1.101; 95% CI=1.004–1.209; p=0.04), respectively (Table 4). Thus, these variables (co-administered drugs and use of PI) were considered risk factors, since increasing the number of co-administered drugs and using PI-containing regimens increased the possibility of developing DIs. The inclusion of a non-ARV drug increased the chance of DIs in 12.9% (95% CI=11.1–14.7%) and the inclusion of PI in the ARVs regimen increased the probability of DIs in 10% (95% CI=0.4–20.9%).
Comparison of demographic and clinical characteristics in 304 patients with and without drug–drug interactions followed at a referral Infectious Diseases Service in Belo Horizonte, 2015–2016.
| Patients with DDIs (n=152) | Patients without DDIs (n=152) | OR (95% CI) [p-value] unadjusted | OR (95% CI) [p-value] adjusted | |
|---|---|---|---|---|
| Sexc | ||||
| Male – n (%) | 97 (63.8) | 105 (69.1) | 1.084 (0.670–1.753) [0.743] | – |
| Female – n (%) | 54 (35.5) | 45 (29.6) | ||
| Age (years)d | ||||
| Median number (interquartile range) | 39 (40–46) | 43 (33–51) | 1.0282 (1.008–1.049) [0.003] | – |
| Number of co-administered drugs | ||||
| Median number (interquartile range) | 4 (2–6) | 0 (0–1) | 2.59b (2.086–3.217) [<0.001] | 1.129 (1.111–1.147) [<0.001] |
| ARV | ||||
| IP and booster RTV – n (%) | 112 (80.6) | 86 (80.4) | 1.581 (0.987–2.534) [0.056] | 1.101 (1.004–1.209) [0.04] |
| No IP and booster RTVn (%) | 21 (19.4) | 21 (19.6) | ||
| CD4+ (cells/mm3)e | ||||
| Median number (interquartile range) | 617 (422–767) | 566 (319–806) | 1.000 (0.999–1.000) [0.254] | – |
| HIV viral load (copies/mL)f | ||||
| <40 | 112 (80.6) | 86 (80.4) | 1.013 (0.536–1.913) [0.968]a | – |
| >40 | 27 (19.4) | 21 (19.6) | ||
Viral load was used as a proxy for estimating the impact of DIs in ARVs effectiveness. The global effectiveness of ARV therapy was 80.5% (VL<40copies/mL). Only 48 patients (19.5%) had a VL above 40copies/mL. Even among participants with seven or more DIs, VL was undetectable for 88.9% of them. CD4+ count was another parameter used to estimate the impact of DIs in ARVs effectiveness. Among those with any DI, 78 (58.6%) had a CD4+ count higher than 500cells/mm3 compared to 76 participants (65.0%) without any DIs. There was no significant impact of the number of DIs on ARV effectiveness (p=0.078).
DiscussionTo our knowledge this is the first study conducted in Brazil that evaluated drug interactions among PLWH and possible repercussions on ARVs effectiveness. Half of the participants (50%) were exposed to at least one DI. Others studies5,8,9 conducted in Iran, India and United States reported similar results (69.9%, 65%, and 41.2%, respectively). Five percent of the DIs were clinically significant interactions, in contrast to that found by Chaitanya10 in India, where 51.3% of the interactions were considered significant. This wide discrepancy can be probably justified by the different software used to classify the severity of interactions and by the difference of the non-ARV drugs taken by the participants.
Four hundred and sixty-two DIs with ARVs were identified and EFZ was the antiretroviral with the most interactions (20.2%), 6.9% clinically significant and 21.1% potentially significant. Until 2017, EFZ-based regimen was recommended in Brazil as the preferred ARV regimen. Such regimen is both inducer and inhibitor of CYP. The results of the present study were similar to the findings of Holtzman et al.11 who also used the University of Liverpool database to characterize the extent of polypharmacy for the interactions between ARV and benzodiazepines and antidepressants in 3080 participants. However, that study found lower rates of DI between lipid reducing drugs, erectile dysfunction drugs and between ATV and lithium carbonate. In addition, a higher prevalence (61%) of drug interaction between omeprazole and ATV was found, compared to 27.1% in the current study.
A retrospective five-year follow up study12 to evaluate prescriptions for multiple medications in PLWH and non-PLWH reported that 35% of PLHW had taken five or more drugs. Another Canadian study13 found 32% of patients on polypharmacy. The lower frequency found in the present study (24%) may be due to underreporting in medical records or reliance on the memory of patients to report the drugs in use. It is also possible that the patients included in this study needed less medication due to better health care.
Patients older than 50 years had more drug interactions with non-ARV drugs (66%) compared to those younger than 50 years (45%) and the latter used less drugs compared to the former [21.5% vs 29.2%]. These results are similar to Marzolini et al.14 who stressed the increasing number of elderly adult PLHW and co-morbidities. It has been shown that chronic infection with HIV and possibly its treatment are contributors to the acceleration of the aging process when compared to those without this condition. Effros et al.15 also pointed that the success of HAART has dramatically enhanced life expectancy of HIV-infected individuals. In 2015, more than one-half of all HIV-infected individuals in the United States will be over 50 years and age influences the course of HIV infection. Dyslipidemia, glucose intolerance, decrease in bone density and osteopenia, hypogonadism, renal and hepatic disorders, psychiatric diseases, neurocognitive impairment, and coronary artery disease are all conditions that are likely to occur earlier in PLWH compared to non-HIV-infected people.16 These aforementioned conditions lead to an increase in the number of medications needed as well as changes in the their pharmacokinetics and pharmacodynamics, increasing the inherent risk for deleterious interactions.17 Participants aged less than 50 years had the lowest number of interactions (83.4%) compared to patients over 50 years (16.0%). This result is close to that found by Katende-Kyenda et al.18 in a study carried out in South Africa evaluating the prevalence of possible DIs with ARV in different age groups. Nine hundred and sixty DIs were reported, 32.40%, 60.21%, and 0.63% for patients <40 years, >40 and ≤60 years, and >60 years, respectively. Patients aged >40 and ≤60 years had the highest number of DIs, whereas patients older than 60 years had the lowest.
Marzolini et al.14 analyzed drug prescriptions for 1497 HIV-infected individuals: 477 age ≥50 and 1020 age <50. Older patients were more likely to receive one or more co-medications compared with younger patients (82% versus 61%; p<0.001) and thus had more frequent DIs (51% versus 35%; p<0.001). Also this result is close to that found in the present study.
Although some drug interactions that inhibit CYP system enzymes and increase ARV metabolism may affect ARVs plasma concentration and reduce its effectiveness, in the current study there was no statistically significant difference in VL among participants who had one or more drug interactions. Marzolini et al.14 had a similar finding. As in our study, Farhoudi et al.5 also identified the number of co-medications and the regimen with PI as independent factors associated with DIs. Similarly, in the study by Holtzman et al.11 older age, number of co-medications, and PI-based ARV regimen were independently associated with DIs. The same finding was reported by Miller et al.9
In conclusion, half (50%) of our patients receiving ARVs were exposed to at least one DI. The three most common clinically significant DIs were omeprazole+ATV (21.7%), simvastatin+ATV and RTV (both 13%), and phenobarbital+DRV (13%). The number of co-administered medications and PI-based ARV regimen were independent factors for DIs.
Agents that promote suppression of gastric acidity may interfere with drug absorption, as gastric pH below seven is an important determinant of solubility and absorption, and consequently the bioavailability of ARVs).19 Another issue that must be taken into account is the fact that the treatment of gastro-esophageal reflux disorder in PLWH can be problematic, as some ARVs require acidic medium for action. A study conducted20 in PLWH in England reviewed information on the frequency and reason for using gastric acid reducing agents, checking for possible drug interactions and whether the risk was documented or mitigated. Seven hundred and one patients on highly active antiretroviral therapy (HAART) were investigated and 67 (69%) were using gastric acid-reducing agents, the most prescribed being proton-pump inhibitors (88.1%). Four potential drug interactions were identified, which were properly managed with a change in the time of administration of ARVs and proton-pump inhibitors in order to increase intervals between doses. When co-administered with ATV, omeprazole should not exceed the dose of 20mg daily and should be administered 12h prior to administration of ATV and RTV.21
The interactions between anti-epileptics and ARVs are considered to be risky associations, as they can cause drug toxicity. In case of such association, it is recommended to monitor therapeutic serum levels of both ARVs and anti-epileptics, in order to obtain an adequate control of seizures, as well as to avoid toxicity.22
Simvastatin, as well as lovastatin, because of their CYP3A4 metabolism, and to a lesser extent atorvastatin, which is only partially metabolized by CYP3A4, are the HMG-CoA reductase inhibitors with the greatest risk of drug interactions and should not be used in patients under HIV therapy. Patients receiving HMG-CoA reductase inhibitors should be monitored regularly for the occurrence of muscular adverse effects and drug interactions should be considered with each new prescription or change in clinical status.
A recent published Brazilian Guidelines23 for the treatment of HIV infection has made available safer and less toxic ARV through the public health system. One of the modifications was the substitution in the initial regimen of EFZ for dolutegravir. Also ATV is not among the first choices for PI-based ARV regimens. This shall mitigate some of the most significant risks for DIs.
This study has limitations. These include the possibility of underestimation of the prevalence of drug interactions as they were dependent on the completeness of medical records and on the patients’ capability of recalling the possible DIs symptoms or signs. Also data on liver and renal function and other metabolic abnormalities were not included because many results were not available to the researchers.
Conflicts of interestThe authors declare no conflicts of interest.